Chapter 12 – The Laws of Thermodynamics
advertisement
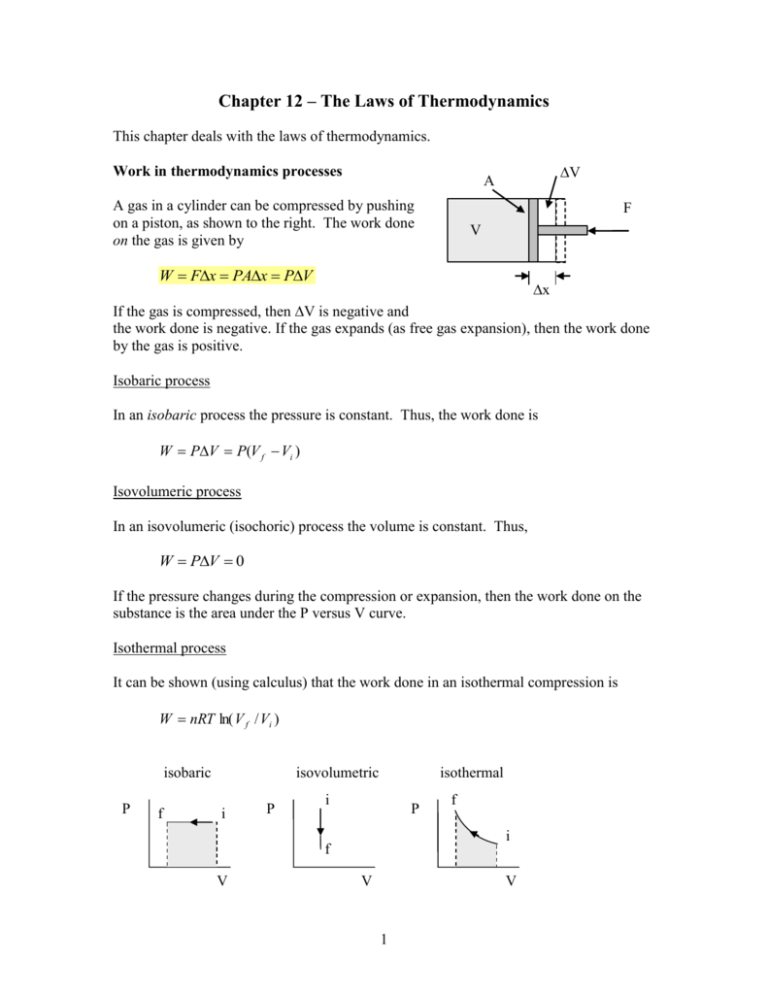
Chapter 12 – The Laws of Thermodynamics This chapter deals with the laws of thermodynamics. Work in thermodynamics processes V A A gas in a cylinder can be compressed by pushing on a piston, as shown to the right. The work done on the gas is given by F V W Fx PAx PV x If the gas is compressed, then V is negative and the work done is negative. If the gas expands (as free gas expansion), then the work done by the gas is positive. Isobaric process In an isobaric process the pressure is constant. Thus, the work done is W PV P(V f Vi ) Isovolumeric process In an isovolumeric (isochoric) process the volume is constant. Thus, W PV 0 If the pressure changes during the compression or expansion, then the work done on the substance is the area under the P versus V curve. Isothermal process It can be shown (using calculus) that the work done in an isothermal compression is W nRT ln( V f / Vi ) isobaric P f isovolumetric i P isothermal i P i f V f V V 1 First Law of Thermodynamics The first law of thermodynamics relates the change in internal energy to the heat absorbed and the work done on a substance. It is essentially a statement of conservation of energy. U U f U i Q NET WNET Q is positive if heat is absorbed and is negative if heat is lost by the system. For a monatomic ideal gas, U 32 Nk BT 32 nRT . Thus, for an isothermal expansion or compression, U 32 nRT 0 , and Q = W. An adiabatic process is one for which no heat flows into or out of a system. For an adiabatic process, U = W. Example: P (kPa) An ideal monatomic gas goes through the cyclic process ABCA as shown to the right. The temperature of the gas at A is 600K. Calculate the work done on the gas, the heat absorbed by the gas, and the change in internal energy for each process and for the total cycle. 30 A 20 10 B C 0 From the ideal gas equation, PV = nRT, we 0 2 can calculate that TB = 600K and TC = 200K. Since this is a monatomic gas, then we have U = 3/2 nRT. We keep in mind that 1 liter = 10-3 m3. V (L) 4 6 AB: Since the gas is expanding, then the work done on the gas is negative and WAB = area under PV curve = (2x104 Pa)(4x10-3 m3) = 80 J UAB = 3/2 nRTAB = 0 (TA = TB) From the 1st law, QAB = UAB + WAB = 0 + 80 J = 80 J (heat is absorbed) 2 BC: Since the gas is being compressed, the work done on the gas is negative and since the area under PV curve = (1x104 Pa)(4x10-3 m3) = 40 J WBC = -40J UBC = 3/2 nRTBC = 3/2 nR(TC – TB) = 3/2 PCVC – 3/2 PBVB =3/2 (1x104 Pa)(2 – 6 )x10-3 m3 = -60 J QBC = UBC + WBC = -60 J+ (– 40) J = -100 J CA: This is an isovolumeric process, so WCA = 0. UCA = 3/2 nRTCA = 3/2 nR(TA – TC) = 3/2 PAVA – 3/2 PCVC =3/2 (3x104 Pa – 1x104 Pa)(2x10-3 m3) = 60 J QCA = UCA + WCA = 60 J Summary results: U = QNET - WNET AB BC CA NET W (J) +80 -40 0 40 Q (J) 80 -100 60 40 0 -60 60 0 U (J) Note that the total work done in the cycle is the enclosed area. The negative value means that the gas does work on its environment during the cycle. Since the gas returns to its original state, the net change in internal energy is zero. The net heat absorbed is equal to the net work done by the gas. Heat is absorbed during the processes AB and CA and rejected during the process BC. P (kPa) 30 Qin area = work 20 10 B C Qout 0 V (L) 0 3 Qin A 2 4 6 Heat Engines and the 2nd Law of Thermodynamics The gas undergoing the cyclic process described in the above problem is an example of a heat engine. It absorbs heat at high temperatures, dumps heat at low temperatures, and converts the difference into work. Qin P Hot Area = work Qin Qout W Qout V Cold The efficiency of a heat engine is the ratio of the net work done during the cycle to the heat absorbed. Since ΔU=0 it follows QNET = WNET with QNET = Qin + Qout = Qin - |Qout|. The efficiency of the heat engine is given by e W Qin Where W is do work done by the system = WNET e Qin Qout Qin 1 | Qout | Qin The 2nd law of thermodynamics states that no heat engine can have an efficiency that is 100% (e = 1). In other words, a heat engine cannot extract heat from a reservoir and convert it completely to work. Some heat must be dumped at lower temperatures. Example: In the previous example of the ideal monatomic gas undergoing a cyclic process, calculate the efficiency. Qin Q AB QCA 80 J 60 J 140 J W (net ) 40 J (done b y the gas ) e W 40 J 0.286 28.6% Qin 140 J 4 Maximum possible efficiency (Carnot heat engine) The maximum possible efficiency of a heat engine that absorbs heat at Thot and dumps heat at Tcold is e 1 Tc Th This would apply if the heat engine were an ideal gas. All other heat engines (Diesel, Otto, etc...) with ideal gases have a lower efficiency than the Carnot cycle. Example: A heat engine absorbs heat at 500oC and dumps heat at 25oC. What is the maximum efficiency? e 1 Tc 25 273 1 0.386 Th 500 273 If the engine takes in heat at the rate of 10 kW, what is the power output? W e Qin Power Qin W e (0.368)(10kW ) 3.68kW t t In reality, since friction would be involved, more heat is actually lost by the heat engine (at each transformation) and the real efficiency is less: e real engine < e ideal engine Entropy The entropy of a system is a measure of its disorder. The higher the disorder, the higher the entropy. Specifically for a system which absorbs heat at a fixed temperature, then the change in entropy is given by S Q T units = J/K or cal/K If heat is absorbed, then S > 0. If heat is lost, then S < 0. 5 Example: 50 g of water melts at 0oC. What is the change in entropy of the water? S Q mL f (50 g )(80cal / g ) 14.7 cal / K 61.3 J / K T T 273K Example: Heat of 100cal is transferred from an object at 100°C to another at 0°C. Find the total change in entropy. Qhot = -100 cal Qcold = 100cal negative sign since this heat is released positive sign since this heat is absorbed 6 Example: 100 cal of heat is transferred from a reservoir at 100oC to a reservoir at 0oC. Assuming that the reservoirs are large enough so that their temperatures don’t change, what is the total change in entropy of the two reservoirs? S S hot S cold Qhot Qcold 100cal 100cal Thot Tcold 373K 273K 0.098 cal / K Note that S > 0, which means that the total disorder has increased. If heat flowed from the cold to the hot, then S would be negative. This cannot occur spontaneously. Another way of stating the 2nd law of thermodynamics is to say that the total entropy of a closed system increases in all natural processes. 7