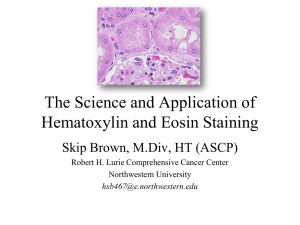
Hematoxylin and Eosin Stain
advertisement

Hematoxylin and Eosin Stain Modified to detect amyloid deposits in frozen sections This stain is based on the observation that amyloid plaques can be revealed with H&E stain even though the dyes are not specific for amyloid. Plaques in paraffin H&E sections resist the stain and appear white on a light pink background, while plaques in frozen sections take up the stain and appear dark pink on a light pink background. Because this protocol was optimized as a counterstain for DAB-developed immunochemistry, we have intentionally reduced the hematoxylin concentration by 10-fold from the original Hopkins Neuropathology recipe. The diluted stain produces an even, although faint, lavender/blue color in the nuclei. The eosin will give the entire tissue a pale pink background; amyloid plaques can be distinguished by their intensity. Hematoxylin Solution The best hematoxylin I have ever used is based on a homemade formula from the Hopkins Neuropathology labs. This solutions really does need to be ‘matured’ in a sunny window for 30 days before use, but its worth it. Add each chemical one at a time, stirring thoroughly after each. Make sure each ingredient is fully dissolved before adding the next. Stir for an additional 2 hours once all components are added, then allow the stain to ripen in sunlight for at least one month prior to use. The final color of the stain should be reddish-violet. Store at room temp., shelf life is approx 6 mo. 0.5 g 500 ml 0.1 g 25 g 0.5 g 25 g Hematoxylin (Sigma #H9627) H2O Sodium Iodate (Sigma #S4007) Ammonium Aluminum Sulfate (Malinckrodt #3212) Citric acid (Malinckrodt #0627) Chloral hydrate (Sigma #C8383) NOTE: Class IV restricted substance, requires DEA license to purchase 2 drops Glacial acetic acid NOTE: When used as a counterstain, this stain should be diluted 5-10-fold in acid alum (the base without the dye) Eosin/Phloxine B I also include here Hopkins’ eosin/phloxine B recipe, although we have gotten good results with a commercially available ready-to-use stain from Medical Chemical Corp. in Torrance, CA, catalog # 554A. Stock A: 1% Eosin 0.5 g Eosin Y (Fisher #E511-25) 50 ml H2O Stir until dissolved, then add: 390 ml 95% Ethanol (made from 100%, non-denatured ethanol) Stock B: 1% Phloxine B 0.5 g Phloxine B (Fisher #P387-25) 50 ml H2O Working Eosin/Phloxine B solution: Make fresh from stocks before use. 390 ml Stock A (1% Eosin) 5 ml Stock B (1% Phloxine B) Stir 5 min, then add: 2.5 ml Glacial acetic acid Stir additional 5 min, then ready to use. Procedure 1. Mount free-floating sections onto subbed slides (i.e. Fisher Superfrost Plus) one day prior to staining. Tissue should be floated onto the slides from a salt-free buffer such as 0.1 M Tris or 0.1 M phosphate buffer, pH 7.4. Let the sections dry onto the slide overnight at RT. 2. Filter the hematoxylin through a fluted Whatman paper prior to use. Every time! 3. Rehydrate the sections in running lukewarm tap water for 15-30 minutes. Thorough rehydration will improve the evenness of the final stain. 4. THIS STEP MAY NOT BE NECESSARY, but we’re not sure: Defat the sections by dehydration through an ascending alcohol series (70%, 95%, 100%) into xylene, then return through descending alcohols (100%, 95%, 70%) into running water. 5. Stain slides for 15-25 minutes in diluted hematoxylin. 6. Destain sections in running tap water for 5-15 minutes. 7. Counterstain slides in eosin/phloxine B for 15-30 minutes. Start by dipping slides into and out of eosin stain several times to coat evenly, then immerse for the remainder of the incubation. 8. Destain through 2 changes of 70% EtOH for 5-10 min each, then rinse away the remaining excess stain in running tap water for another hour or so until the background is pale and the plaques are dark pink. This step may not be optimal, and might be improved by decreasing the time in eosin or going straight to water after staining. The one solid data point I have is that differentiating in 95% EtOH as recommended in the standard protocol removed the eosin from both the plaques and the background and so was not a good idea when trying to stain in a way that highlighted the deposits. I also found that there was no improvement by adding acid to the 70% alcohol during differentiation. Best with a short stint in regular 70% EtOH and then into water for a long long time. 9. Dehydrate through an ascending alcohol series into xylene and coverslip with Permount.