ggge20730-sup-0001-2015GC005779-SupInfo
advertisement

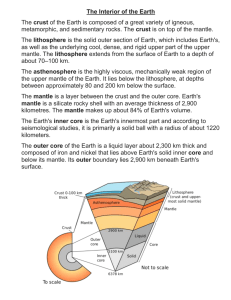
Geochemistry, Geophysics, Geosystems Supporting Information for Plume-cratonic lithosphere interaction recorded by water and other trace elements in peridotite xenoliths from the Labait volcano, Tanzania Hejiu Hui1,2,3, Anne H. Peslier4, Roberta L. Rudnick5, Antonio Simonetti2, Clive R. Neal2 1State Key Laboratory for Mineral Deposits Research, School of Earth Sciences and Engineering, Nanjing University, Nanjing 210023, China 2Department of Civil and Environmental Engineering and Earth Sciences, University of Notre Dame, Notre Dame, IN 46556, USA 3Lunar and Planetary Institute, USRA – Houston, Houston, TX 77058, USA 4Jacobs, NASA Johnson Space Center, Mail Code XI3, Houston, TX 77058, USA 5Department of Geology, University of Maryland, College Park, MD 20740, USA Contents of this file Text S1 Figures S1 and S2 Additional Supporting Information (Files uploaded separately) Captions for Tables S1 to S4 Introduction These supplementary materials provide additional information for analytical methods, melting and melt-rock interaction models, and effective viscosity calculation. In addition, trace element data are included in the supplementary tables. The authors consider the additional information important as it clarifies the discussion in the main text. 1 Text S1. 1. Analytical Methods Portions of the mantle xenoliths were gently crushed to keep grain integrity. Around 30 (or as many as we could obtain) representative mineral grains for olivine, orthopyroxene, clinopyroxene, and garnet were handpicked in ethanol under a binocular microscope. Selected mineral grains were washed with ethanol and dried at room temperature overnight. The dried mineral grains were subsequently embedded in epoxy resin. Doubly polished sections 70 to 300 m thick were prepared using an Ingram grinder and an automatic polisher with diamond paste (down to 1 m). The sections were cleaned with acetone and deionized water. 1.1. FTIR Analyses Each mounted mineral grain was examined under a petrographic microscope. Two criteria were used to select grains for Fourier transform infrared spectrometer (FTIR) analysis: (1) the minerals should contain zones that are optically free of fractures and inclusions [Bell et al., 1995], (2) for biaxial minerals (olivine, orthopyroxene and clinopyroxene), the polished surface should be oriented perpendicular to the optic normal (ON), acute bisectrix (Bxa) or obtuse bisectrix (Bxo) optical axes. The grain crystallographic orientation was identified with interference figures and recorded. Garnet is uniaxial, and thus the crystal orientation does not affect its infrared spectra. The thickness of each selected grain was measured using a Mitutoyo digital micrometer. The grain mounts were stored overnight in a cabinet under a N 2 flux before FTIR analyses. Polarized infrared spectra of olivine and pyroxene crystals with wave numbers from 4000 to 650 cm-1 were collected using two different FTIRs at NASA-Johnson Space Center: A Continuµm microscope attached to a Nicolet NEXUS 670FT FTIR spectrometer, and later a newly installed Hyperion 3000 microscope attached to a Bruker Vertex 70 FTIR spectrometer. The mercury cadmium telluride (MCT) detector and KBr beam splitter were used during FTIR analyses. A Zn-Se wire grid polarizer rotated parallel to the optical indicatrix , or , was used during analyses of olivine, orthopyroxene and clinopyroxene. Aperture size from 23 23 to 100 100 µm2 during each analysis was chosen based on the grain size and the distribution of fractures and inclusions in the grain. Each analysis typically consisted of 256 scans performed in a N2 gas environment, thus minimizing interference from atmospheric water vapor. Up to four different locations in each grain were analyzed to check the homogeneity of water within the grain. Typically a new background spectrum was collected before a new grain was analyzed. Cross-sectional analyses on two olivine grains were also carried out to demonstrate the homogeneity of water within the grain using an AutoImage microscope on a Perkin Elmer Spectrum GX FTIR at the University of Michigan using the same experimental procedure as already described. Each spectrum was normalized to 1 cm thickness and, if necessary, the baseline was manually corrected using the Nicolet Omnic software. The overall absorption intensity in the O-H region was determined by integrating the area beneath the OH absorbance bands (between 3600 and 3000 cm-1 for olivine and garnet, 3700 and 2800 cm-1 for orthopyroxene, and 3700 and 3000 cm-1 for clinopyroxene). The integrated absorption intensity was converted to water content (ppm H2O, and hereafter) using the Beer-Lambert law in the form CH2O = As/I, where for pyroxene and olivine As = A + A + A is the sum of the integrated absorption intensities for three principle directions (cm-2) (A, A and A correspond to the integrated absorbance beneath the OH band with the vector of the incident infrared light E//, // and //, respectively) while for garnet, it represents the integrated area beneath OH bands of 2 unpolarized spectra. I is the integral molar absorption coefficient (ppm-1cm-2) of 5.320.34 for olivine, 14.840.59 for orthopyroxene, 7.090.32 for clinopyroxene, and 1.390.14 for garnet [Bell et al., 1995, 2003]. Uncertainties are generated at each step of the data acquisition and reduction processes. The uncertainty in grain orientation is estimated to be 5, which converts to 5% H2O. The error from mineral mount thickness measurement is 3 µm. The largest uncertainty comes from the manual correction of spectrum baseline using Omnic software. The quantification of this uncertainty is somewhat ambiguous due to subjective characteristics of identification of the baseline, which can be up to 10%. The overall uncertainty (1) of water contents determined is typically up to 20%, calculated for each water content using an error propagation formula. 1.2. LA-ICP-MS Trace Element Analyses Trace- and minor-element concentrations of orthopyroxene, clinopyroxene and garnet from the mantle xenoliths were measured using a 213 nm Nd-YAG New Wave laser ablation system in conjunction with a Thermo-Finnigan Element 2 high resolution magnetic sector inductively coupled plasma mass spectrometry (LA-ICP-MS) in the Midwest Isotope and Trace Element Research Analytical Center (MITERAC) at the University of Notre Dame. The CaO concentration of each mineral from the previous electron probe microanalysis [Lee and Rudnick, 1999] was used as an internal standard (CaO weight percent measured by the electron microprobe, and 43Ca abundances measured by LA-ICP-MS). Silicate glass NIST 612 was used as an external standard to correct for instrument drift and ensure reproducibility in the measurements. Due to the low concentration of most trace elements in orthopyroxene, the largest aperture (250 m) was used to ensure maximum material ablated for analyses in the mass spectrometer. For clinopyroxene and garnet, different apertures (40, 80, 100 or 125 m) were used in order to obtain accurate measurements of incompatible trace elements with low concentrations, such as LREEs. The laser had a repetition rate of 5 Hz, and 100% power output. Each spot analysis consisted of approximately 20 s collection of background (gas blank) and approximately 40 s of laser ablation on the sample. The ablated particles were transported into the ICP-MS by a helium-argon gas mixture with 0.7 l/min of He. The LA-ICP-MS data were reduced using GLITTER (a software for the laser ablation microprobe, which provides the realtime interactive data reduction for LA-ICP-MS analysis). Furthermore, GLITTER can be used to monitor whether incompatible-trace-element rich inclusions were ablated during analysis as sudden change of concentrations of incompatible trace elements and Ca can be identified in the time-resolved data. No inclusions appear to have been ablated during our analyses. Typically, three grains, if possible, were analyzed to check the grain heterogeneity within each mantle xenolith. The propagated standard errors (1) of 58 analyses of the external standard during the measurement are better than 0.2 ppm for all the trace elements except Be (0.29 ppm), Ti (0.23 ppm), and Sr (0.36 ppm). Variability of orthopyroxene trace element contents within one xenolith is less than an order of magnitude (mostly <50%), except for Cu, Mo, Sb, Yb, and Pb in LB45, Ba in KAT17, Th in LB1, Pb, Ce, and Ba in LB11, Rb, Mo, Ba, and Lu in LB53, and Rb, Ba, Dy, and Pb in LB24 (Table S1). 1.3. Trace Element Analysis Comparison with those of Aulbach et al. [2008] Our new measurements of trace element abundances in clinopyroxenes from KAT17 and LB4 are consistent with those from Aulbach et al. [2008] (Fig. S1). However, trace element contents of LB45 clinopyroxene from Aulbach et al. [2008] are slightly higher than those 3 obtained in this study (Fig. S1). This discrepancy could be due to sample heterogeneity, perhaps linked to the presence of different generations of metasomatic clinopyroxene. 2. Melting and Melt-Rock Interaction Models Three models are used in the present paper to quantify the behavior of water in mantle processes (Fig. S2): equilibrium melting, fractional melting, and melt-rock interaction (MRI). (1) Equilibrium batch melting [Shaw, 1979; Norman, 1998], 0 (S1a) CS = (DS/L ´ CBR ) /(DS/L ´ XS ´ (1- F) + F), BR/L 0 BR/L (S1b) CBR = (D ´ CBR ) /(D ´ (1- F) + F), where CS and XS are the water content and mass fraction of olivine, orthopyroxene, or clinopyroxene respectively, DS/L is the partition coefficient of water between mineral phase (olivine, orthopyroxene, or clinopyroxene) and silicate melt, CWR0 is the initial whole-rock water content, CWR is the whole-rock water content, DWR/L is the partition coefficient of water between peridotite and silicate melt, and F is the fraction of silicate melt to total mass during partial melting. (2) Fractional melting [Shaw, 1979; Norman, 1998], 0 (S2a) CS = (CWR / XS ) ´ (1- F)1/(D ´XS )-1, 0 WR/L 1/ P -1 (S2b) CWR = CWR ´ (1- (P ´ F /D )) , i i/L where P = å p D , i can be olivine, orthopyroxene, and clinopyroxene [Shaw, 1979]. Here pi S/L i is the mass fraction of each mineral in silicate melt [Shaw, 1979] and assumed to be constant in our calculation, 0.3 for clinopyroxene, 0.1 for orthopyroxene and 0 for olivine. (3) Melt-rock interaction [DePaolo, 1981], (S3a) Ca = (CDM ´ DWR/L ) /(DWR/L ´ (1- F) + F), 0 -1 0 (S3b) Cm = Cm ´ f (1- (Ca /Cm ) ´ (r /(r -1))), (S3c) CBR = Cm ´ Mm + Ca ´ (1- Mm ), Ol, Opx CCpx = (CWR - CWR /D WR/L ´ åM i ´ Di / L ) / M Cpx, (S3d) åM i ´ Di / L ) / M Opx , (S3e) ´ Di / L ) / M Ol , (S3f) i Ol, Cpx COpx = (CWR - CWR /DWR/L ´ i Opx, Cpx COl = (CWR - CWR /DWR/L ´ åM i i where CDM is the water concentration in the depleted mantle, Cm0 is the initial concentration of water in the melt that interacted with the depleted mantle (0.5 wt% and 0.25 wt%), f (assumed to be 0.95) is the mass ratio of the interacted magma to the initial magma; r (assumed to be 0.97 [Kelemen et al., 1998]) is the mass ratio of the assimilated magma to the crystallized magma, Mm is the proportion of the interacted magma added into the peridotite (1 and 0.5 wt% of magma containing 0.5 and 0.25 wt% H2O respectively), and Mi (i = Ol, Opx, Cpx) is the modal proportion of each mineral phase in peridotite. Water contents of both depleted mantle (DM = 150 ppm H2O, near upper limit of MORB source [Michael, 1988; Dixon et al., 2002]) and primitive mantle (PM = 1100 ppm H2O [Palme and O’Neill, 2007]) were used in our modeling (Fig. S2). The modal proportion used for clinopyroxene, orthopyroxene, and olivine in the equilibrium and fractional melting models are 15%, 25%, and 60% respectively, while those in the melt-rock interaction modeling are 10%, 4 25%, and 65% respectively. The partition coefficients of water between pyroxenes and melt were calculated using the equations of O’Leary et al. [2010] using the chemical compositions of LB45 pyroxenes, which is the most fertile sample [Chesley et al., 1999]. A constant value of 0.0006 [Tenner et al., 2009] for water partitioning between olivine and melt is used. The water partition coefficient between bulk peridotite and melt was assumed to be 0.008 (at 2 GPa [Tenner et al., 2009]). 3. Effective Viscosity Calculation Effective viscosity (eff; in Pas) is defined as the ratio of the shear stress (; in Pa) to the strain rate ( e˙ ; in s-1): (S4) heff = t /e˙. The shear stress in our calculation is assumed to be 0.3 MPa. The strain rate of an olivine aggregate can be calculated using following equation [Mei and Kohlstedt, 2000]: e˙ = Acret n1(exp(c 0 + c1 lnCOH + c 2 ln 2 COH + c 3 ln3 COH )) r exp(- Q + PVcre ), RT (S5) where Acre = 90MPa-(n1+r)s-1 with the experimentally derived stress exponent n1 = 3.5 and the water fugacity exponent r = 1.2 [Hirth and Kohlstedt, 2004], Q is the activation energy (480 kJ/mol [Hirth and Kohlstedt, 2004]), Vcre is the activation volume (10.610-6 m3/mol [Li et al., 2008]), and COH is the water content in H per 106 Si converted from that in ppm using a SiO2 content of 40.27 wt% in LB45 olivine [Lee and Rudnick, 1999]. The temperature T in K varies with the depth in km and was calculated using two different cratonic geotherms. One based on a typical Archean cratonic geotherm with a surface heat flow of 41 mWm-2 [Rudnick et al., 1998] is: (S6) T = 88.304 + 7.057P - 0.0096P 2 + 5.7 ´10-6 P 3. The other one using a geotherm passing through the xenolith equilibration temperatures and pressures with a surface heat flow of 50 mWm-2 [Lee and Rudnick, 1999] is: (S7) T = -22.192 +16.495P - 0.0752P 2 + 20.4 ´10-5 P 3. References Aulbach, S., R. L. Rudnick, and W. F. McDonough (2008), Li-Sr-Nd isotope signatures of the plume and cratonic lithospheric mantle beneath the margin of the rifted Tanzanian craton (Labait), Contrib. Mineral. Petrol., 155, 79-92. Bell, D. R., P. D. Ihinger, and G. R. Rossman (1995), Quantitative analysis of trace OH in garnet and pyroxenes, Am. Mineral., 80, 465-474. Bell, D. R., G. R. Rossman, J. Maldener, D. Endisch, and F. Rauch (2003), Hydroxide in olivine: A quantitative determination of the absolute amount and calibration of the IR spectrum, J. Geophys. Res., 108, doi: 10.1029/2001JB000679. Chesley, J. T., R. L. Rudnick, and C.-T. Lee (1999), Re-Os systematics of mantle xenoliths from the East African Rift: Age, structure, and history of the Tanzanian craton, Geochim. Cosmochim. Acta, 63, 1203-1217. DePaolo, D. J. (1981), Trace element and isotopic effects of combined wallrock assimilation and fractional crystallization, Earth Planet. Sci. Lett., 53, 189-202. Dixon, J. E., L. Leist, C. Langmuir, and J.-G. Schilling (2002), Recycled dehydrated lithosphere observed in plume-influenced mid-ocean-ridge basalt, Nature, 420, 385-389. Hirth, G., and D. L. Kohlstedt (2004), Rheology of the upper mantle and the mantle wedge: A view from the experimentalists, Geophys. Monograph Ser., 138, 83-106. 5 Kelemen, P. B., S. R. Hart, and S. Bernstein (1998), Silica enrichment in the continental upper mantle via melt/rock reaction. Earth Planet. Sci. Lett., 164, 387-406. Lee, C.-T., and R. L. Rudnick (1999), Compositionally stratified cratonic lithosphere: Petrology and geochemistry of peridotite xenoliths from the Labait tuff cone, Tanzania, Proc. Intern. Kimberlite Conf., 7, 503-521. Li, Z.-X., C.-T. Lee, A. H. Peslier, A. Lenardic, and S. J. Mackwell (2008), Water contents in mantle xenoliths from the Colorado Plateau and vicinity: Implications for the mantle rheology and hydration-induced thinning of continental lithosphere, J. Geophys. Res., 113, doi: 1029/2007JB005540. Mei, S., and D. L. Kohlstedt (2000), Influence of water on plastic deformation of olivine aggregates 2. Dislocation creep regime, J. Geophys. Res., 105, 21471-21481. Michael, P. (1988), The concentration, behavior and storage of H2O in the suboceanic upper mantle: Implications for mantle metasomatism, Geochim. Cosmochim. Acta, 52, 555-566. Norman, M. D. (1998), Melting and metasomatism in the continental lithosphere: laser ablation ICPMS analysis of minerals in spinel lherzolites from eastern Australia, Contrib. Mineral. Petrol., 130, 240-255. O’Leary, J. A., G. A. Gaetani, and E. H. Hauri (2010), The effect of tetrahedral Al3+ on the partitioning of water between clinopyroxene and silicate melt, Earth Planet. Sci. Lett., 297, 111-120. Palme, H., and H. St. C. O’Neill (2007), Cosmochemical estimates of mantle composition, Treat. Geochem., 2, 1-38. Rudnick, R. L., W. F. McDonough, and R. J. O’Connell (1998), Thermal structure, thickness and composition of continental lithosphere, Chem. Geol., 143, 395-411. Shaw, D. M. (1979), Trace element melting models, Phys. Chem. Earth, 11, 577-586. Tenner, T. J., M. M. Hirschmann, A. C. Withers, and R. L. Hervig (2009), Hydrogen partitioning between nominally anhydrous upper mantle minerals and melt between 3 and 5 GPa and applications to hydrous peridotite partial melting. Chem. Geol., 262, 42-56. 6 Figure S1. Comparison of trace element abundances in clinopyroxenes from KAT17, LB45, and LB4 obtained in this study and by Aulbach et al. [2008]. 7 Figure S2. Equilibrium, fractional melting and melt-rock interaction (MRI) models for the Labait peridotites. See Fig. 1 caption for details on the modeling. 8 Table S1. Trace elements (ppm) of orthopyroxene from Labait mantle xenoliths. Table S2. Trace elements (ppm) of clinopyroxene from Labait mantle xenoliths. Table S3. Trace elements (ppm) of garnet and kelyphite from Labait mantle xenolith LB24. Table S4. Water contents in melt equilibriated with olivine, orthopyroxene, and clinopyroxene of Labait mantle xenoliths. 9