CHEM 5013
advertisement

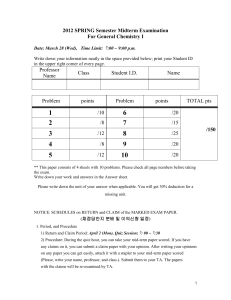
CHEM 5013 REVIEW SHEET EXAM #2 Chapter Six – The Periodic Table and Atomic Structure [6.2] Wave Theory of Light – Know the definitions of frequency, wavelength, amplitude of a wave [6.2] Electromagnetic Spectrum – Understand the relationships between frequency and wavelength. You need to know the formula c=λν. You also need to know how to convert meters to nanometers (nm). I will provide the value of c (Speed of light in a vacuum). [6.2] Energy (particle) Theory of Light – Understand the relationships between frequency, wavelength, and energy. You need to know the formula E=hν. I will provide the value of Planck’s Constant (h). [6.3] Atomic Spectra – Definitions of Continuous Spectrum and Line Spectrum, Ground State and Excited State (Spec 20 Lab) (6.4) Quantum Numbers – We discussed four different ones – These are the addresses or locations of electrons (6.4) Analogy: Road or street = Shell, House = Subshell, Room = Orbital (6.4) Limitations on number of subshells in a shell or on number of orbitals in a subshell or number of electrons in an orbital (6.5) Pauli Exclusion Principle – Understand and be able to apply to a given orbital diagram or electron configuration (6.5) Write the Complete Electron Configuration for an Element using Building Up Principle (Aufbau’s Principle) or the Periodic Table (6.5) Write an Electron Configuration Using the Periodic Table – What are the different “blocks” on the table? (6.5) Draw an Orbital Diagram for an Element (6.5) Definitions of Valence Electrons vs. Core Electrons [6.5] Write the Shorthand Electron Configuration for an Element (Noble Gas Core) (6.5) Understand and Apply Hund’s Rule to Provide the Lowest Energy Level Configuration of Electrons in an Element [Lab] You need to know the formula ABS=2-log(%T) and how to use it. [Lab] There will be some type of question where you will have to interpret a plot of %T vs. wavelength to determine the likely color of a material as in the Spec-20 lab. [Lab] There will also be some type of question where you will have to use a Beer’s Law Plot to estimate the concentration of a solution of a chemical as you did in lab. You WILL NOT have to perform linear regression analysis. Chapter Seven – Chemical Bonding and Molecular Structure (7.2) Ionic Bonds are formed between Metals and Non-Metals (Cations and Anions) [7.2] Use the Periodic Table to determine the charges on ions and how they react with one another [7.2] Use Lewis Diagrams of elements and ions to pictorially show the transfer of electrons to form an Ionic bond and ions of Noble Gas configuration [7.3] Lewis Electron Dot Symbols of Elements and Ions [7.3] Covalent bonds are formed between non-metals and involve the sharing of electrons equally between both elements [7.3] Definition of bonding pairs and lone pairs of electrons [7.3] Use Lewis Diagrams of elements to pictorially show how a covalent bond is created in a molecule by sharing electrons between atoms [7.5] Draw Lewis Structures for various molecules and polyatomic ions Chapter Three – Chemical Formulas and Aqueous Solutions [3.2] Definitions – Chemical Equations, Chemical Reactions, Reactants, Products [3.2] Balancing chemical equations – Law of conservation of matter – Understand that you balance by changing the coefficients NEVER the subscripts!!! [3.3] Aqueous Solutions o Define: solution, solute, solvent, solubility, soluble, insoluble o Understanding of solubility tables – YOU WILL BE GIVEN A SOLUBILITY TABLE but you must know how to interpret it o Define and give examples of: Electrolytes, Non-electrolytes, Strong Electrolytes, Weak Electrolytes, Spectator Ions Rules for Exam 1. NO CALCULATORS ARE ALLOWED. YOU WILL NOT NEED THEM. 2. You will be provided with a reference sheet and periodic table identical to those on the website 3. NO OTHER outside information may be used. Advice Study the suggested practice problems and quizzes – many problems will be similar to the problems on these assignments. Study your lecture notes and textbook sections provided above. Valuable Hint: Chemistry is a subject which builds upon itself. You will need to use some information that you learned for Exam 1 in order to solve some problems on Exam 2. Specifically, it is assumed that you know how to provide correct chemical formulas given the name of a compound and that you can provide the name of a compound given the chemical formula. It is also assumed that you will know how to convert from one Metric system unit to another (ex. mL to cm3 or mL to L) Example: You should be able to provide a correct formula for calcium carbonate (CaCO3) NO CALCULATORS ARE ALLOWED FOR THIS EXAM! YOU WILL NOT NEED THEM.