Using up a metal
advertisement

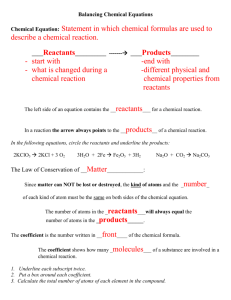
Using up a metal Purpose: To examine a chemical reaction and to relate this chemical reaction to the conservation of atoms and of matter Materials: Copper, 8 Molar Nitric acid, Clay, Toothpicks, a tube with a cap, and one Digital balance Data table: Mass # 1: Tube + Copper + Nitric acid (not combined): ________ Mass #2: Tube + copper + Nitric acid (Once the reaction has reached an end) __ Procedure: Part I: The reaction 1. Record the mass of the tube, nitric acid and copper by placing all three objects in a cup. (Be sure to tare he balance first, and to place the tube so it is upright) 2. Open the tube, drop the copper in, and close the tube. 3. Record observations for what you see happening (I expect three sentences detailing three changes that you see take place 4. Place the reaction tube on the balance and record the post reaction mass. Part II: The model Examine the equation that represents the reaction you have just carried out: Cu + 4 HNO3 Cu(NO3)2 + 2 H2O +2NO2 1. Take the clay and make “atoms” to represent the reactants. You will need 4 colors (one for copper, one for hydrogen, one for nitrogen and one for oxygen. Be sure you make only enough “atoms for the reactants (one copper, 4 hydrogen, 4 nitrogen, and 12 oxygen atoms) 2. Use toothpicks to connect the atoms into molecules – sketch the reactants (4 molecules, and one atom of copper) 3. Once you have sketched the reactants, pull the molecules apart – but do not destroy any atoms. Use as many toothpicks as you need to rearrange your atoms into the formula units and molecules of the products. (Hint it is easier if you remove the hydrogen, but leave the NO3 as a unit, until you make the Cu(NO3)2 ) 4. Sketch the models you have made , Use color pencils to match the clay colors you have used Questions 1. Where did the metallic copper go during the reactions? In the end; what state (solid, liquid gas or aqueous) could you find it in? 2. Consider the colored gas. What atoms were in these molecules, and where did these atoms come from. (your answer must be one or more than one of the reactants) 3. Consider the water named as a product. Where did these hydrogen and oxygen atoms originate (Your answer must be one or more of the reactants) 4. Consider the measurements of mass that you made using the digital balance. How did the mass of the reactants compare to the mass of the products. What would Dalton have said concerning your results? Conclusion: What could you conclude from this experiment concerning the underlying nature of chemical reactions?