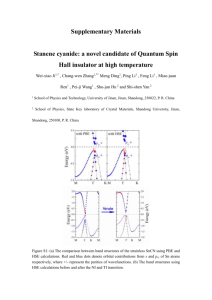
jssc3808-sup-0001-FigureS1
advertisement

Supporting Information Dicationic imidazolium ionic liquid modified silica as a novel reversed-phase/anion-exchange mixed-mode stationary phase for high performance liquid chromatography Min Sun, Juanjuan Feng*, Xiaojiao Wang, Huimin Duan, Leilei Li, Chuannan Luo** Key Laboratory of Chemical Sensing & Analysis in Universities of Shandong (University of Jinan), School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China *Correspondence: Dr. Juanjuan Feng, Key Laboratory of Chemical Sensing & Analysis in Universities of Shandong (University of Jinan), School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China; E-mail: chm_fengjuanjuan@ujn.edu.cn (J. Feng); Fax: +86-536-89736065 **Correspondence: Prof. Chuannan Luo, Key Laboratory of Chemical Sensing & Analysis in Universities of Shandong (University of Jinan), School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China; E-mail: chm_luocn@ujn.edu.cn (C. Luo); Fax: +86-536-89736065 Reagents Spherical and porous silica particles of 6 µm, with 8 nm average pore diameter and 390 m2 g−1 specific surface area (BET), which were obtained from Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences (Lanzhou, China), were used as the support. 3-Mercaptopropyltrimethoxysilane (98%) was obtained from Qufu Chenguang Fine Chemical Co. (Shandong, China) and purified by vacuum distillation before use. 1-Vinylimidazole (98%), 1,6-dibromohexane (97%) and azobisisobutyronitrile (AIBN) were purchased from Shanghai Jingchun Industry Co. (Shanghai, China). 1-Vinylimidazole and 1,6-dibromohexane were purified by vacuum distillation before use. AIBN was purified by recrystallization in ethanol before use. Toluene was dried by refluxing with sodium for 24 h, and then distilled before use. Polycyclic aromatic hydrocarbons (PAHs), phenols, anilines, organic acids, ethanol and methanol used in chromatographic separations were analytical reagents; inorganic salts including potassium bromate, potassium bromide, potassium nitrate, potassium iodide and potassium thiocyanate were analytical reagents. Other organic and inorganic compounds used in experiments were of analytical grade quality and were used without further purification. Synthesis of dicationic imidazolium ionic liquid Synthesis of 1,1’-(1,6-hexanediyl)bis(1-vinylimidazolium) bibromide was according to the following procedures: 1-vinylimidazole (9.42 g, 0.10 mol) was dissolved in 40 mL acetone; 1,6-dibromohexane (7.78 g, 0.05 mol) was dissolved in 10 mL acetone and slowly added into the 1-vinylimidazole solution. The mixture was stirred for 24 h at room temperature. The precipitate was obtained by filtering the liquid phase. The product was purified by recrystallization in acetone three times. The structure was confirmed by 1H NMR. 1H NMR (DMSO-d6, 400 MHz; δ, ppm, relative to TMS): 9.429–9.436 (t, 1H), 8.180–8.189 (t, 1H), 7.891–7.899 (t, 1H), 7.236–7.297 (q, 1H), 5.909–5.954 (q, 1H), 5.408–5.435 (q, 1H), 4.151–4.187 (t, 2H), 1.792–1.826 (t, 2H), 1.265–1.300 (t, 2H). Characterizations 1 H NMR (UNITY INOVA-400 MHz) was used to confirm the formation of dicationic imidazolium bromide salt. Surface properties of SilprSH and Sil-DIL were characterized by a field-emission scanning electron microscope (SEM, SUPRATM55, Carl Zeiss, AG, Germany) and an energy-dispersive X-ray spectrometer (EDS, Oxford INCA X-Act, England). The carbon, hydrogen and nitrogen contents of SilprSH and Sil-DIL were determined by elemental analysis, performed on an Elementar Vario EL cube (Hanau, Germany). The average concentration of mercaptopropyl groups and IL groups can be calculated through the carbon content of SilprSH and nitrogen content of Sil-DIL, respectively. The specific surface area (BET) of silica, SilprSH and Sil-DIL was investigated on an ASAP 2010 Accelerated Surface Area and Porosimetry System (Micromeritics, USA). The calculating formulas are as follows: (1) Mercaptopropyl groups on SilprSH (µmol m−2) = (2) IL groups on Sil-DIL (µmol m−2) = C % 106 = 2.85 36 S1 N % 10 6 = 0.25 56 S 2 (1) (2) where C% and N% represent the percentage of carbon and nitrogen, respectively. S1 and S2 are the specific surface area of silica (390 m2 g−1) and SilprSH (330 m2 g−1), respectively. Fig. S1. The EDS spectra of SilprSH (a) and Sil-DIL (b). Fig. S2. The effect of methanol content on retention factors (k) of PAHs (a) and anilines (b), respectively. Chromatographic conditions: mobile phase: methanol-water, other conditions are the same as in Fig. 2. Fig. S3. The effect of KH2PO4 concentration (a) and pH (b) of the mobile phase on the retention factors (k) of inorganic anions containing bromate, bromide, nitrate, iodide and thiocyanate. Chromatographic conditions: mobile phase: the solution of KH2PO4, detection: UV at 200 nm, other conditions are the same as in Fig. 2. Fig. S4. The chromatogram of the inorganic anions containing bromate (1), bromide (2), nitrate (3), iodide (4) and thiocyanate (5). Chromatographic conditions: mobile phase: 150 mmol L-1 KH2PO4 solution (pH=4.6), detection: UV at 200 nm, other conditions are the same as in Fig. 2.