CHEMISTRY
advertisement

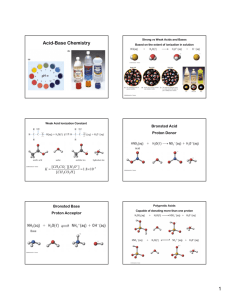
CHEMISTRY MRS. SINGH CHAPTER 19 NOTES SXN. 19.1 Acids H+ ions Taste sour Sharp taste(lemons) Bases OH- ions Taste bitter Feel slippery (soap) Definition of acids & bases: 1. Arrhenius Acid = substance that contains hydrogen and ionizes in aqueous solution to produce hydrogen ions Ex. HBr(g) ↔ H+(aq) + Br-(aq) Base = substance that contains a hydroxide group and dissociates in aqueous solution to produce hydroxide ions NaOH(s) ↔ Na+ (aq) + OH-(aq) 2. Bronsted-Lowry Acid = hydrogen-ion donor; what remains is the conjugate base Base = hydrogen-ion acceptor; what forms is the conjugate acid Ex. NH3(aq) + H2O(l) ↔ NH4 (aq)+ + OH(aq) base acid conjugate conjugate acid base Ex. Do # 2 on pg. 599 Monoprotic acid = an acid that can donate only one hydrogen ion Ex. HCl(aq) + H2O(l) ↔ H3O+(aq) + Cl- (aq) Polyprotic acid = acids that can donate more than one hydrogen ion Ex. Write the steps in the complete ionization of boric acid H3BO3 1 Amphoteric = substances which can act as both acids and bases Ex. H2O Anhydrides = oxides which become acids or bases by adding water Ex. CO2(g) + H2O(l) → H2CO3 (aq) Nonmetal oxide + water = acid Ex. CaO(s) + H2O(l) → Ca+2(aq) + 2OH-(l) Metal oxide + water = base SXN. 19.2 Strong acid = an acid that ionizes completely in dilute aqueous solution All strong acids have weak conjugate bases HX(aq)+ H2O(l) →H3O+(aq) + X-(aq) Copy strong acids & ionization equations from table 19-1 on pg. 603 Weak acid = an acid that ionizes only partially in dilute aqueous solution Any acid that is not a strong acid is a weak acid! Ka = acid ionization constant Ex. HCN(aq) + H2O (l) ↔ H3O + (aq) + CN- (aq) Keq = Do: #10 on pg. 605 2 SXN. 19.3 The Dissociation of Water Since water is an amphoteric substance, it can act as an acid or a base depending on what is dissolved in it. CO32–(aq) + H2O → HCO3–(aq) + OH–(aq) Water is acting as an acid since it is donating a proton to the carbonate ion. HCl(aq) + H2O → H3O+(aq) + Cl–(aq) Water is acting as a base since it is accepting a proton from hydrochloric acid. H+ (aq) + OH– (aq) H2O (l) H2O (l) + Acid – Conjugate Base Pair H3O+ (aq) + OH–(aq) H2O (l) Base – Conjugate Acid Pair When water itself dissociates, it is acting as an acid and base at the same time. The K in the dissociation of water shown above is very small. It is also always the same number if the temperature remains constant, say 25°C. It is called the ION – PRODUCT CONSTANT. Kw = [H3O+] [OH–] = [H+] [OH–] = 1.0 x 10–14 A neutral solution is where [H+] = [OH–] An acidic solution is where [H+] > [OH–] A basic solution is where [H+] < [OH–] The pH(power of Hydrogen) scale measures the degree of acidity of a solution and is based on common logarithms. The pH scale is a measurement of the concentration of hydrogen ions [H+]. The numbers on the pH scale are the negative logarithm of the [H+]. Since Kw = [H+] [OH–] = 1.0 x 10–14 (at 25°C), the pH scale measures from 0 to 14. [H+] 7 0 1 2 3 4 5 6 8 increasing acidity NEUTRAL 9 10 11 12 increasing alkaline (base) 13 3 14 Since the exponents are negative numbers, the greater the concentration of H+, the smaller the pH number. The lower the pH number, the more acidic the solution. The higher the pH number, the more alkaline. Because pH numbers are powers of ten, an acid of pH = 4 is ten times more acidic than a solution with a pH = 5, and one-hundred times more acidic than a solution with a ph = 6. In a neutral solution, [H+] = [OH–] = 1.0 x 10–7 pH = – log [H+] = – log [10–7] = 7. So a pH of 7 is neutral. pOH = -log [OH-] pH + pOH = 14.00 Ex. The concentration of either the H+ ion or the OH- ion is given for three aqueous solutions at 298 K. For each solution, calculate [H+] or [OH-]. State whether the solution is acidic, basic, or neutral. a. [H+] = 1.0 x 10-4 M b. [OH-] = 1.3 x 10-2 M c. [H+] = 5.8 x 10-11 M The rule for pH is: The number of significant figures the concentration has, is the number of decimal places the pH should have. Ex. Calculate the pH at 298 K of solutions having the following ion concentrations. a. [H+] = 1.0 x 10-6 M b. [H+] = 3.8 x 10-11 M Ex. Calculate the pOH and pH at 298 K of solutions having the following ion concentrations. a. [OH-] = 1.0 x 10-12 M b. [OH-] = 1.3 x 10-2 M 4 Calculating ion concentrations from pH a. What are [H+] and [OH-] in a healthy person’s blood that has a pH of 7.50? Assume temp is 298 K. b. Find the [H+] and [OH-] if pOH = 2.95 Calculating the pH of solutions of strong acids and strong bases For all strong monoprotic acids, the concentration of the acid is the concentration of H+ ion. Ex. HCl(aq) → H+(aq) + Cl-(aq) If you have a 0.1M HCl solution, it contains 0.1 mole of H+ ions per liter and 0.1 mole of Cl- ions per liter Most strong bases behave the same way: Ex. KOH(aq) → K+(aq) + OH-(aq) A 0.5 M NaOH produces 0.5 mol of K+ ions per liter and 0.5 mol of OHions per liter. Ex. What is the pH of a 0.0001 M solution of HCl? Since HCl H+ + Cl–, a 1.0 x 10–4 concentration of HCl produces a 1.0 x 10–4 concentration of H+ ions. So, pH = – log [H+] = – log [1.0 x 10–4] = 4.00 Ex: What is the pH of a 0.00432 M solution of HCl? Ex: What is the pH of a 0.0329 M solution of NaOH? 5 Using pH to calculate Ka Ex. Calculate the Ka for the following acids using the given information: a. 0.220 M solution of H3AsO4, pH = 1.50 b. 0.0400 M solution of HClO2, pH = 1.80 Measuring pH The approximate pH of a solution can be obtained by wetting a piece of pH paper with the solution and comparing the color of the wet paper with a set of standard colors. The pH meter provides a more accurate measurement in the form of a digital display of the pH. SXN. 19.4 NEUTRALIZATION A neutralization rxn is a rxn in which an acid and a base react in aqueous solution to produce a salt and water. A salt is an ionic compound made up of a cation from a base and an anion from an acid. Neutralization is a double-replacement rxn Ex. Mg(OH)2(aq) + 2 HCl(aq) → MgCl2(aq) + 2H2O(l) Note that the cation from the base (Mg the acid (Cl-) in the salt MgCl2 +2 ) is combined with the anion from Do #29 on pg. 617 6