Department of Chemistry - University of Missouri
advertisement

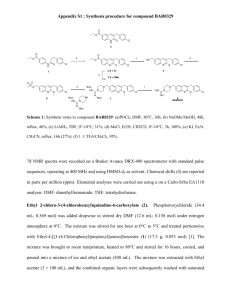
Department of Chemistry University of Missouri-St. Louis Name__________________ Practice Exam Chem 2633 Some additional useful information can be found at the end of the exam 1. A student mixed 10.0 g of 3-methyl-1-butanol with 15 mL glacial acetic acid (100%, density 1.12 g/mL) and 3 mL concentrated sulfuric acid (density 1.84 g/mL) and heated the mixture to boiling for an hour. On cooling, the mixture was added to cold water, the layers were separated in a seporatory funnel and the organic layer was washed with sodium bicarbonate, water and then slurried (mixed) with anhydrous sodium sulfate. After filtering, the organic layer was distilled to yield 8 g of 2-methylbutyl acetate (banana oil). A. Write an overall equation for this reaction. CH3 O CH3 COH + CH3 CH CH2 CH2OH H2O + CH3 CH3 O CH CH2 CH2 O C CH3 B. On the basis of the amounts of reagents used, what is the limiting reagent (show your work)? 3-methyl-1-butanol = 10g/88 g/mol = 0.114 mol LIMITING REAGENT H2SO4 catalyst glacial acetic acid 1.12g/mL*15mL = 16.8; 16.8 g/60 g/mol = 0.28 mol C. Calculate the theoretical yield in grams. MW isoamyl acetate = 130 g/mol; 0.114 mol * 130 g/mol = 14.8 g D. What is the % yield? 8g/14.2g = 0.563 or 56.3% E. What was the purpose of washing the organic layer with NaHCO3? To remove any acids dissolved in the ester F. Why was the organic layer swirled with anhydrous Na2SO4? To dry the ester so that it would distill properly and to remove the possibility of back hydrolyses 1 2.A. Define Rf as used in thin layer chromatography. Rf = distance travelled by the solute/distance travelled by the sovent B. What could you do to increase the Rf value of a particular compound? Increase the polarity of the solvent C. A student spots an unknown and develops it in CH2Cl2. Only one spot is observed with an Rf value of 0.5. Is the compound likely a pure sample? What additional experiment could you do using thin layer chromatography to test your hypothesis? Most likely; use a different solvent or different stationary phase 3.A. How is the solubility of a solid in a given solvent usually affected by decreasing the temperature? Solubity in any solvent generally decreases with decreasing temperature B. What is the mole fraction of toluene in a mixture of 1 mol of cyclohexane (C), 2 mol methylcyclohexane (M)? mol fraction methylcyclohexane = mols of M /(mol of M + mol of C) = mol fraction methylcyclohexane = 2/3 = 0.66 4A If aspirin has a water solubility of 1 gram/100 mL and a solubility in ether of 10 g/100 mL, how much of this material could you ideally dissolve in 200 mL of a 1:1 volume mixture of H2O : ether? 10g + 1 g = 11g B. What is the value of the equilibrium constant, K, for aspirin in the following expression? (g/100 mL)ether = (g/100mL)H2O K = 10/1 2 5. Describe the purpose of the following component of a gas chromatograph. A. Injector: Its purpose is to quickly volatilize the solutes being chromatographed B A student’s gas chromatograph of the natural product in CH2Cl2 recorded the following two peaks: retention times: 2.85 and 5.85 min. The following retention times of the stardards, also run in methylene chloride, were measured: Compound A: 2.86 6.0 Compound B: 3.0 6.0 Compound C: 2.85 5.6 Compound D: 3.0 6.2 Which compound did the student have, A, B, C, or D? relative to the internal standard the student peak eluted at (5.85-2.85 )min = 3.0 min Only compound B has the same retention time relative to the standard. 6. A. What are the melting characteristics of an impure compound? Generally melts lower and over a broader temperature range B. Suggest a purpose for running a mixed melting point (a mixed melting point refers to the process of measuring the melting point of a mixture prepared by mixing a known and unknown compound, when your unknown compound is suspected to be the same as your known)? Explain what you would observe in the melting point if the two compounds are identical or if they are different. Identical: sharp mp Different: broad mp and probably lower Molecular weight of acetic acid: 60; 3-methyl-1-butanol: 88; H2SO4: 98 g/mol 3