3.6 Suspensions and filtration
advertisement
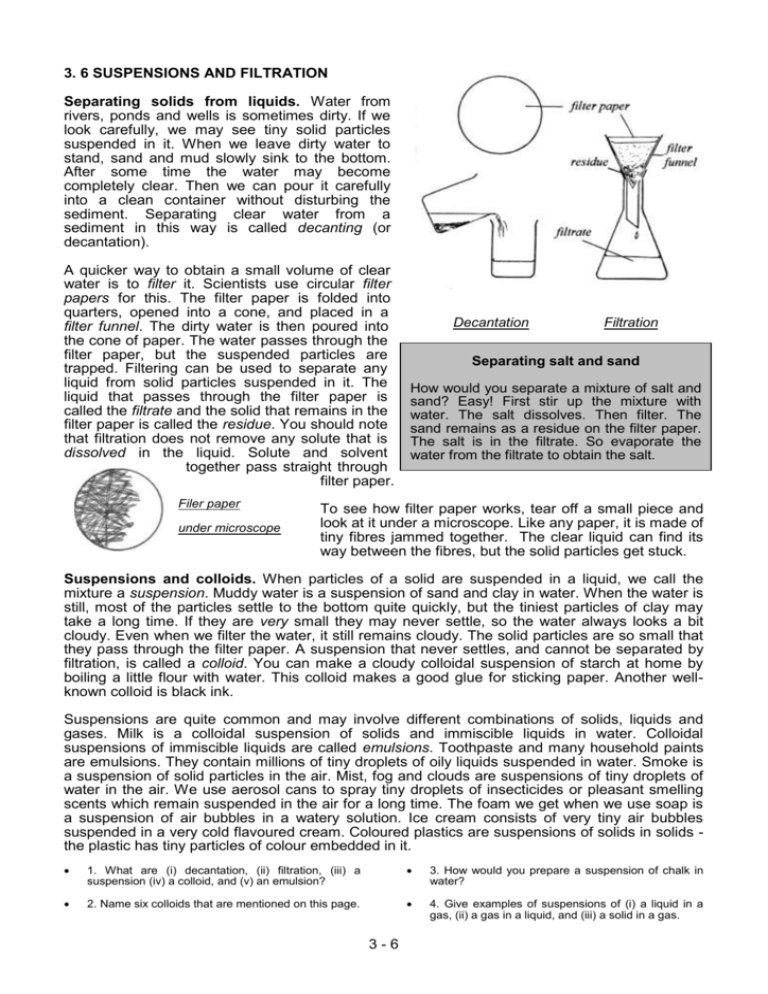
3. 6 SUSPENSIONS AND FILTRATION Separating solids from liquids. Water from rivers, ponds and wells is sometimes dirty. If we look carefully, we may see tiny solid particles suspended in it. When we leave dirty water to stand, sand and mud slowly sink to the bottom. After some time the water may become completely clear. Then we can pour it carefully into a clean container without disturbing the sediment. Separating clear water from a sediment in this way is called decanting (or decantation). A quicker way to obtain a small volume of clear water is to filter it. Scientists use circular filter papers for this. The filter paper is folded into quarters, opened into a cone, and placed in a filter funnel. The dirty water is then poured into the cone of paper. The water passes through the filter paper, but the suspended particles are trapped. Filtering can be used to separate any liquid from solid particles suspended in it. The liquid that passes through the filter paper is called the filtrate and the solid that remains in the filter paper is called the residue. You should note that filtration does not remove any solute that is dissolved in the liquid. Solute and solvent together pass straight through filter paper. Filer paper under microscope Decantation Filtration Separating salt and sand How would you separate a mixture of salt and sand? Easy! First stir up the mixture with water. The salt dissolves. Then filter. The sand remains as a residue on the filter paper. The salt is in the filtrate. So evaporate the water from the filtrate to obtain the salt. To see how filter paper works, tear off a small piece and look at it under a microscope. Like any paper, it is made of tiny fibres jammed together. The clear liquid can find its way between the fibres, but the solid particles get stuck. Suspensions and colloids. When particles of a solid are suspended in a liquid, we call the mixture a suspension. Muddy water is a suspension of sand and clay in water. When the water is still, most of the particles settle to the bottom quite quickly, but the tiniest particles of clay may take a long time. If they are very small they may never settle, so the water always looks a bit cloudy. Even when we filter the water, it still remains cloudy. The solid particles are so small that they pass through the filter paper. A suspension that never settles, and cannot be separated by filtration, is called a colloid. You can make a cloudy colloidal suspension of starch at home by boiling a little flour with water. This colloid makes a good glue for sticking paper. Another wellknown colloid is black ink. Suspensions are quite common and may involve different combinations of solids, liquids and gases. Milk is a colloidal suspension of solids and immiscible liquids in water. Colloidal suspensions of immiscible liquids are called emulsions. Toothpaste and many household paints are emulsions. They contain millions of tiny droplets of oily liquids suspended in water. Smoke is a suspension of solid particles in the air. Mist, fog and clouds are suspensions of tiny droplets of water in the air. We use aerosol cans to spray tiny droplets of insecticides or pleasant smelling scents which remain suspended in the air for a long time. The foam we get when we use soap is a suspension of air bubbles in a watery solution. Ice cream consists of very tiny air bubbles suspended in a very cold flavoured cream. Coloured plastics are suspensions of solids in solids the plastic has tiny particles of colour embedded in it. 1. What are (i) decantation, (ii) filtration, (iii) a suspension (iv) a colloid, and (v) an emulsion? 3. How would you prepare a suspension of chalk in water? 2. Name six colloids that are mentioned on this page. 4. Give examples of suspensions of (i) a liquid in a gas, (ii) a gas in a liquid, and (iii) a solid in a gas. 3-6