Chapter 11 - Spokane Public Schools
advertisement

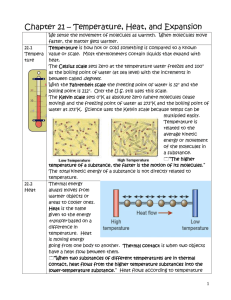
Chapter 11 Temperature, Heat, & Phases of Matter Name ______________________________ 7th grade Science Period ______ 1. Fahrenheit – A temperature scale that has water freezing at 32 degrees, and boiling at 212 degrees, is the Fahrenheit scale. There are 180 Fahrenheit degrees between the freezing point and boiling point of water. The U. S. uses this scale. (pg. 276) 2. Celsius – The Celsius temperature scale has water freezing at zero and boiling at 100 degrees. Most scientists use this scale. To convert Fahrenheit into Celsius and back again use the following formulas: Tfahrenheit = 9/5 Tcelsius + 32 Tcelsius = 5/9 (Tfahrenheit - 32) (pg. 276) 3. Thermometer– An instrument that measures temperature is called a thermometer. (pg. 276) 4. Kinetic Energy – The kinetic theory tells us that tiny particles of matter (atoms and molecules) never stop moving. This energy of movement is called kinetic energy. 5. Thermal Energy – Thermal energy is the energy in an object created by temperature (heat). Adding heat increases the energy of molecules and they move faster, with more motion, and get farther apart (kinetic energy). Cooling takes thermal energy (heat) away and molecules move slower, with less kinetic energy, and get closer together. (pg. 278) 6. Thermal Expansion/Contraction – Matter expands when you add thermal energy (heat) because the molecules get farther apart. This is called thermal expansion. Matter contracts when it is cooled because the molecules get closer together. This is called thermal contraction. 7. Random Motion & Temperature – When kinetic energy (motion) scatters equally in all directions this is called random energy. The random energy of each atom in a substance is called random motion. Temperature is a measurement of the kinetic energy in each atom due to this random motion. Remember that it is the amount of heat that effects the movement of the atoms. (278) 8. Average Motion - Kinetic energy can also be a measurement of the average motion of all the atoms in a substance together. This is not a temperature measurement. It is a speed measurement. Example: When you throw a rock it has velocity (kinetic energy). All of the atoms in the rock are moving together in a certain direction at a certain speed. (pg. 278) 9. Absolute Zero – When molecules have the lowest energy they can have, and the temperature cannot get any lower, this is called absolute zero. Thermal energy (heat) is at its lowest possible levels and is as close to zero as it can be (about -273 degrees Celsius). Note: There is no highest possible temperature. Temperature can go up indefinitely. (pg. 279) 10. Kelvin Scale – The temperature scale that starts at absolute zero is called the Kelvin scale. The Kelvin scale goes up in units identical to the Celsius scale. Water freezes at 273 degrees K, and boils at 373 degrees K. To convert to Kelvin you add 273.16 to the Celsius temperature (21 degrees C + 273.16 = 294.16 degrees K). (pg. 279) 11. Solid – A state of matter that holds its shape and does not flow is called a solid. Solids also hold their volume. Molecules in a solid vibrate in place but do not move far from their positions. Most solids are locked closely together in crystals (a regular, orderly arrangement of atoms). Solids are the most dense forms of matter. Note: Some solids such as glass, many plastics, rubber, and wax appear to be solids but they have no crystals. The atoms have a random and jumbled arrangement. These solids are called amorphous solids. (pg. 280, 314) 12. Liquid – A liquid is a state of matter that holds its volume but will not hold its shape. A liquid flows and easily changes shape. Liquids are about as close together as solids but they have enough energy to move around freely and change positions. They have broken their crystals. (pg. 280) 13. Gas – A gas flows like a liquid but can also expand or contract to fill a container. A gas does not hold its volume. Gas molecules have enough energy to completely spread away from each other and are much farther apart than solid or liquid molecules. (pg. 280) 14. Plasma – At temperatures greater than 10,000 degrees Celsius the atoms of gases begin to come apart forming positive and negative ions. This phase of matter is called plasma. Plasma is formed in lightning and inside stars. (pg. 279) 15. Intermolecular Forces – Forces between molecules that determine which phase of matter they will be are called intermolecular forces. These are weaker forces than the chemical bonds that hold atoms together forming compounds. (pg. 280) 16. Melting Point/Freezing Point – The point at which a solid gains enough heat to melt into a liquid, or a liquid loses enough heat to freeze into a solid. Different substances have different melting and freezing points. When something melts the atoms vibrate more vigorously, break their crystals, and begin to move freely. When something freezes the atoms slow down and lock back into crystals, losing the ability to move freely. They simply vibrate in place. (pg. 281) 17. Boiling Point/Condensation Point – The point at which a liquid gains enough heat energy to boil into a gas, or a gas loses enough heat to condense into a liquid. Different substances have different boiling and condensation points. (pg. 281) 18. Evaporation - When a liquid changes to a gas gradually, at temperatures below the boiling point, this is called evaporation. Evaporation is usually caused from radiation from the sun. 19. Phase Change – As the temperature of solid gains heat energy it reaches melting point and begins to change phase to a liquid. During the phase change temperature stays constant because all of the heat energy is being used to break the solid into a liquid. As the temperature of a liquid rises it will reach boiling point and the liquid will change phase to a gas. Again during the phase change temperature remains constant. (pg. 281) 20. Pressure – Pressure can be used to cause phase changes in gases. Pressure forces molecules closer together. Lack of pressure allows molecules to spread apart. Applying enough pressure to a gas, and cooling it at the same time, can turn it into a liquid, or even a solid. 21. Sublimation - When a solid gains enough thermal energy to change phase directly to a gas, skipping the liquid stage, this is called sublimation. 22. Heat – Thermal energy that is moving or capable of moving is called heat. It takes twice as much energy to heat 2000 grams of water as it does to heat 1000 grams of water. (pg. 283) 23. Joule – The metric unit used to measure heat is called the Joule. (pg. 284) 24. Calorie – One calorie is the amount of heat energy needed to raise the temperature of 1 gram of water 1 degree Celsius. One calorie is equal to 4.186 joules. The unit used to measure the energy of the food we eat is the kilocalorie. A kilocalorie in food equals 1000 calories. Example: a cookie containing 210 food calories contains 210,000 units of heat calories. (pg. 284) 25. British Thermal Unit (Btu) – An amount of energy needed to raise 1 pound of water 1 degree Fahrenheit is called a British thermal unit. One Btu is equal to 1055 joules. (pg. 284) 26. Specific Heat – The amount of heat needed to raise the temperature of 1 kilogram of a material by 1 degree Celsius is called specific heat. The specific heat of water is 4184J/Kg C. The heavier an object is the lower its specific heat. (pg. 286) 27. Heat Transfer – The flow of heat from higher temperature to lower temperature is called heat transfer. (pg. 287) 28. Conduction – The transfer of heat from particle to particle by direct contact is called conduction. 29. Thermal Equilibrium – When things are at the same temperature and no heat flows back and forth between them this is called thermal equilibrium. (pg. 287) 30. Thermal Conductors & Thermal Insulators – Materials that easily conduct heat are called thermal conductors. Materials that conduct heat poorly are called thermal insulators. (pg. 288) 31. Convection – The transfer of heat by the flow (movement) of heated matter is called convection. Hot air rises and cold air sinks causing convection currents. Liquids also act in the same manner. (pg. 289) 32. Thermal Radiation – The transfer of heat by electromagnetic waves (including light) is called thermal radiation. Heat from the sun gets to us by thermal radiation. Unlike conduction and convection thermal radiation can travel through the vacuum of space. It does not need any medium to move through. (pg. 290)