Heat and Temperature
advertisement

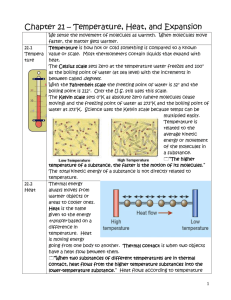
Heat and Temperature (Adapted from http://science.howstuffworks.com/dictionary/physics-terms/heat-info3.htm) Heat or thermal energy is most intense in substances whose molecules are moving rapidly. (Remember rapidly moving molecules = heat) Such substances will give up some of its heat to another nearby substance whose molecules are moving less rapidly. (Slow moving molecules = coolness) When this happens, the heat is said to “flow” from one substance to another (or from one body to another). The energy transfer is indicated by a change in temperature. Temperature, therefore, is not the same thing as heat—although the two words are often used interchangeably. In general terms, temperature can be defined as the degree of intensity of hotness or coldness. Scientists say that temperature is the average kinetic energy in an object. Thermal energy is a form of kinetic energy. In the Energy simulation, you saw the energy symbols for thermal energy. When heated, the iron block had more thermal energy; thus, its temperature was higher. As it cooled, it lost some of its thermal area to its surroundings, so it had a lower temperature. Another way to think about it is that temperature is the ability of one thing to give up heat energy to another thing. In the simulation, the hot brick becomes cooler, and the water becomes warmer, as long as heat is flowing from one to the other. The hot brick has a greater ability to give up heat and therefore has a higher temperature. Some of its thermal energy flowed to the water causing it to heat up (more thermal energy.) After a time the two things reach a condition of “heat equilibrium,” or balance of heat intensity. When this happens, the heat flow stops. At this point (of equilibrium) both things are at the same temperature. Look back at the simulation. Compare the thermometers (which measure the amount of thermal energy in an object) of the heated brick in the water after a minute or two. Did you notice that they were the same? As Isaac would say, the 2nd Law of Thermodynamics says, “Heat moves in a predictable flow from warmer objects to cooler objects until all objects are at the same temperature.” Heat and Thermal Energy (adapted from http://www.physics4kids.com/files/thermo_intro.html) When scientists originally studied thermodynamics, they were really studying heat and thermal energy. Heat can do anything: move from one area to another, get atoms excited, and even increase energy in a system. Did we say energy? That's what heat is. When you increase the heat in a system, you are really increasing the amount of energy in the system. Simply put, the study of thermodynamics is the study of the amount of energy moving in and out of systems. Remember that a system can be anything from a weather box or cup of water to an ecosystem, or the Earth’s atmosphere. Heat of Atoms Energy is moving around the world. You need to remember that it all happens on a really small scale. Energy that is transferred is at an atomic level. Atoms and molecules are transmitting these tiny amounts of energy. When heat moves from one area to another, it's because millions of atoms and molecules are working together. Those millions of pieces become the energy flow throughout the entire planet. In our “Weather in a Jar” activity, water was boiled giving it more thermal energy. This energy fueled a convection current in the jar. As the tiny molecules of water were moving faster, they changed from liquid water to water vapor. As this water vapor rose away from the warm water, it cooled and started to condense. But cooling really just means that the heat left. Where did the heat go? Heat Movement Heat moves from one system to another because of differences in the temperatures of the systems. If you have two identical systems with equal temperatures, there will be no flow of energy. When you have two systems with different temperatures, the energy will start to flow. Areas of high temperature give off energy to areas with lower temperature. There is a constant flow of energy throughout the universe. Heat is only one type of that energy. Listen to Sycorax! KE is an abbreviation for kinetic energy.