urine dosage
advertisement

Stamp (Address): Identification of patient Initials of Name, Surname: _____ ______ Contact person: ________________________________ Tel : ( _______ ) ___________________ Fax: _________ E-mail: __________________________________________ Sex: m f Date of birth: ____. _____. ________ Weight: ______ kg European Study Group for HUS and related disorders 5th year checkup HUSNET (CQ_IBK_aHUS_05 / version 25/11/09) Medical University Innsbruck Department Pediatrics I Prof. Dr. L. B. Zimmerhackl Dr. Johannes Hofer, Dr. Magdalena Riedl, Dr. Alejandra Rosales Tel. +43/512/504-23501 Fax +43/512/504-25450 E-mail: johannes.hofer@i-med.ac.at, magdalena.riedl@i-med.ac.at, alejandra.rosales@i-med.ac.at E-mail: lothar-bernd.zimmerhackl@uki.at HUS related symptoms? yes no ? If yes, give details _________________________ CNS symptoms yes no ? Which? ________________________________ Art. hypertension yes Proteinuria: no ? Genetics FACTOR H Gene FACTOR I CD 46/MCP Factor B Thrombomodulin C3 CFHR1/3 ADAMTS13 Antibodies N N N N N N N N N path path path path path path path path path ? ? ? ? ? ? ? ? ? Creatinine (blood): _______ mg/dl ______µmol/l Creatinine Clearance: ______ ml/min/x1,73m2 (Schwartz) Hb: _____ g/l Platelets: _______ x103/µl LDH: ______ U/l Haptoglobin: ______g/l C3: ____mg/dl C4 : ____ mg/dl Other complement proteins: _________________ yes Urine diagnostics: no ? Reduced renal functions: yes no ? Other diseases?: ________________________________________ Relaps in the last year? yes no number________ HD Date of investigation: ____.____.______ Date of diagnosis: ____.____.______ Labor diagnostics: Date: ____________ Clinical course since 2nd year check up: Dialysis Height: _______ cm PD no transplantation? yes no date:_____________ Sample urine (24h) _____ ml: yes no Spoturine: yes no Stix: Protein: ________ Erys: ________ Proteinuria: ______ g/l _____ g/g Creatinine Creatinine ______ g/l Haematuria: ______ cells/µl Others: _________________________________ Diagnostics: Blood pressure RR: ____/____ mmHg, RR: ____/____ mmHg, RR: ____/____ mmHg yes no pathological: yes no * Renal sonography: studied: yes no * If pathological, please send the attach as a copy! pathological: yes no * 24 h- blood pressure: studied: Continuing drug therapy?: Diuretics: Anti-hypertensive: Anti-epileptics: Calcineurine inhibitors: MMF: Steroids: Rituximab: IVIG: yes no yes no yes no yes no yes no yes no which: _______________ dosage: _____________________ yes no which: _______________ dosage: _____________________ yes no which: _______________ dosage: _____________________ which: _______________ dosage: _____________________ which: _______________ dosage: _____________________ which: _______________ dosage: _____________________ which: _______________ dosage: _____________________ which: _______________ dosage: _____________________ Plasma exchange: _____________ (ml/kg/week) FFP Octaplas others weekly or__________ Plasma infusion: ______________ (ml/kg/week) FFP Octaplas others weekly or ____________ Other therapy: ____________________________________________________ Notes: Characterisation of relapses: Relapse No Date BP (sys/diast) Proteinuria (Albustix) Creatinine clearance (Schwartz formula) 1 2 3 4 5 6 7 Treatment of relapses: ________________________________________________ ____________________________________________________________________ ____________________________________________________________________ Outcome of transplantation number (___) Patient alive Graft failure Number of recurrences If dead, date Graft failure If yes, due to HUS recurrence due to rejection due to other cause no no yes Yes ___/___/___ no Cause yes no no no yes yes yes If yes, please specify ___________________________________________________________________





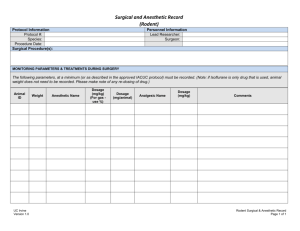



![Detection of proteinuria[1]](http://s3.studylib.net/store/data/007549979_2-02bd2c299a632d6a55125f2f2a73449c-300x300.png)