esmidtermreview
advertisement

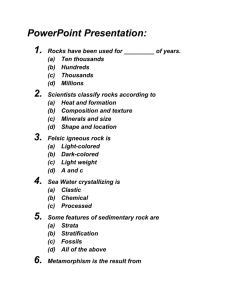
Name____________ EARTH SCIENCE MID-TERM REVIEW CHAPTERS 1 , 2 AND MEASUREMENTS Please define the following terms: System open system Biosphere geosphere Hydrosphere biogeochemical Condensation transpiration model theory law scientific method Independent variable dependent variable closed system atmosphere evaporation carbon sinks hypothesis scientific notation density Fill in the “chart” regarding the metric system please. Concept Definition Official Metric Unit Tool of Measurement Length Mass Time Volume Temperature Skills To Know: Labeling of the water cycle Identify systems as open or closed Identify which of four spheres something belongs to Scientific notation Metric conversions Density problems Draw and label the water cycle. List and describe Earth’s four spheres. Give examples of things in each sphere. Identify each as an open or closed system. Explain why. A pool with kids jumping in and out— The earth— A volcano— A sealed jar of water— Put each measurement into scientific notation please. 4 000 000 m ______________ 425 m____________________ 0.000 045 m__________________ 0.052 m______________________ Put each measurement into standard notation please. 4.6 x 10 4 _____________________ 3.75 x 10 -3 ____________________ 4.3 x 10 0______________________ Make the following metric conversions using the dimensional analysis method please. 250 kg =_____________hg 0.003 mg =____________cg 30 dam =____________dg 655 cg =______________dg Solve the following density problems please. What is the density of a 25 kg object whose volume is 2.3 L? What is the volume of a 25 cg object whose density is 0.75 cg/mL? What is the mass of a 2500 mL object with a density of 1.5 g/mL? Name_______________ EARTH SCIENCE MID-TERM REVIEW CHAPTERS 3 AND 4 Define the following terms please. Equator prime meridian Meridians hemisphere Topography contour map Map globe Compass rose radar Aerial photography rotation Mantle lithosphere Crust inner core Autumnal equinox vernal equinox Winter solstice rotaion Parallax Coriolis effect Elliptical orbit parallels cartographer contour interval GPS plane-table surveying revolution asthenosphere outer core summer solstice revolution oblate spheroid Skills to Know: Map projections—three types, disadvantages, advantages Use of latitude and longitude Reading/interpreting a contour map Draw and label diagram of Earth’s interior Evidence of rotation Evidence of revolution Seasons—reasons, durations, daylight vs dark hours List the three types of map projections. Define each (how is it made). Give the advantages and disadvantages of each. Latitude/Longitude: Draw and “label” a globe. Mark and label the equator and three lines of latitude and longitude. Review practice sheets on latitude and longitude. Contour maps: What are the “rules” for contour maps? How do you find the contour interval? What does it look like when the slope gets steeper? When it flattens? Draw a picture of a contour map and label each of these scenarios. Review practice sheets on contour maps. Draw and label a diagram of the earth’s interior. List the evidences of the earth’s rotation. List the evidences of the earth’s revolution. What is a solstice? An equinox? What do each mean regarding the numbers of daylight and dark hours. When does each occur in the Northern Hemisphere? Name___________________ EARTH SCIENCE MID-TERM REVIEW MATTER UNIT Define each term please. matter compounds heterogeneous metalloids ionic bond two types of matter two types of mixtures metals ions covalent bond Give the symbols for each element please. Sulfur____________ Hydrogen______________ Silver____________ Gold__________________ Nickel___________ iron___________________ Nitrogen__________ silicon_________________ Copper____________ phosphorus_____________ Iodine_____________ chlorine________________ Bromine___________ lead___________________ Mercury___________ zinc___________________ Potassium__________ tungsten________________ Sodium____________ neon___________________ elements homogeneous nonmetals octet rule metallic bond Helium_______________ Cobalt________________ magnesium_____________ oxygen_________________ calcium_________________ aluminum_______________ fluorine_________________ chromium_______________ tin_____________________ carbon__________________ Tell if each is a pure substance or a mixture please: Ice cream____________ blood__________ Table salt____________ vegetable soup____________ Water_______________ tap water_________________ Quartz_______________ silver____________________ Gold________________ air_______________________ Tell if each mixture is homogenous or heterogeneous please: Taco_____________ air____________________ Sprite____________ ranch dressing___________ Tell if each is a metal, nonmetal, or metalloid please: Silver___________ sodium______________ Hydrogen___________ silicon_______________ Boron______________ aluminum_____________ Tell the symbol of the element at each location please: Group 1, Period 4 _______________ Period 5, Group 16_______________ Period 3, Group 14_______________ Give the number of protons, neutrons, and electrons in a neutral atom of each please. Oxygen-18 p__________ n___________ e____________ Hydrogen-2 p__________ n___________ e____________ Alumimun-27 p__________ n___________ e____________ Give the name and charge for the ion of each element please. Sodium ______________ __________________ Nitrogen ______________ __________________ Sulfur ______________ __________________ Magnesium ______________ __________________ Tell if the bond that occurs between each is most likely to be ionic, covalent, or metallic. Gold and gold__________________ Silver and chlorine_______________ Oxygen and fluorine______________ Sodium and bromine______________ Hydrogen and oxygen____________ List the covalent prefixes please. Give the missing name or formula for each covalent compound please. N2O5______________ dihydrogen monoxide_______________ NO3_______________ tetracarbon decahydride______________ PCl3_______________ sulfur trioxide___________________ OF2_______________ phosphorus pentabromide____________ List the steps to writing ionic formulas please. Give the missing name or formula for each ionic compound please. Al2O3_______________ sodium oxide____________________ MgCl2_______________ calcium fluoride__________________ CaS_________________ sodium nitride____________________ NaCl_________________ magnesium sulfide________________ Name_________________ EARTH SCIENCE MID-TERM REVIEW CHAPTERS 5, 6, and 7 (There is no review guide for Chapter 8 because we just did Chapter 8!) Define the following terms please: Luster cleavage Hardness specific gravity Rock magma felsic magma mafic magma Sedimentary rocks metamorphic rocks Stratification intrusive igneous rock Pluton batholith Sill conservation Nonrenewable resources recycling streak characteristics of minerals lava igneous rocks rock cycle extrusive igneous rock dike renewable resources Skills to Know: Be able to identify characteristics of the minerals from our class lab. Be able to identify characteristics of the rocks from our rock lab. Be able to classify minerals into their families. Be able to classify rocks into their families. Draw and label the rock cycle. Minerals from class: Galena— Sulfur— Gypsum— Talc— Mica— Calcite— Pyrite— Quartz— Rocks from class: List each rock from our class lab and briefly describe it. Mineral Groups: List minerals in each group please. Silicates— Native elements— Carbonates— Oxides— Sulfides— Rock Groups: List rocks in each group please. Igneous: Granite— Gabbro— Diorite— Sedimentary: Clastic— Chemical— Organic— Metamorphic: