Lab mass, volume of solids
advertisement

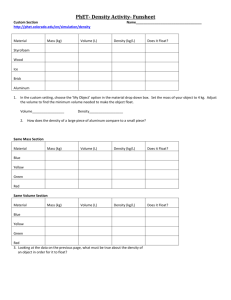
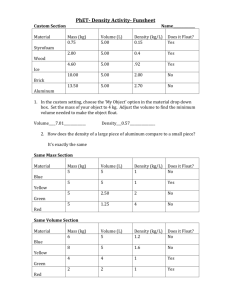
Lab: Relating Mass and Volume of SOLIDS Purpose: 1. To determine the relationship between mass, volume and the mass:volume ratio of different solids 2. To determine a method of how to predict if a solid will float in water. Materials: Various Solids Centigram balance Graduated cylinder + Water Millimeter ruler Procedure: 1. Determine the mass and volume of each of the solids. Record this in the chart below. 2. Place each solid into the water to see if it will float. Record this in your chart. 3. Calculate the mass/volume ratio for each solid. Record the ratio in your chart. (divide the mass by the volume and round off to one decimal place) Observations: Copy the chart into your lab report: Solid Mass (g) Aluminum Cube (silver colored) Iron cube (is rusty) Graphite cube (like the inside of a pencil) Bronze cube (looks more gold colored) Copper cube (looks more penny colored) Copper penny Wax White plastic Cork (beige) Rubber stopper Marble wood Lead Volume (mL or cm3) Mass/Volume Does it float in ratio water? Discussion Questions: 1. What parts of this experiment could have caused experimental error? 2. Compare the Aluminum cube to the copper cube. How does their mass compare? How does their volume compare? How can this be? 3. Compare the Mass/volume ratio column to the “does it float” column. What do you notice about the VALUE of the Mass/volume ratio of all the objects that float? Of all the objects that don’t float? 4. Look back to your lab 4.6 – mass of volume (of liquids). What did you find the mass/volume ratio for distilled water to be? How does that value compare to those of the objects that float? That sink? 5. An unknown object has a mass/volume ratio of 0.4. Do you think that this object would float or sink in water? Explain why. 6. Go to section 4.7 in your textbook. What is the name for the “mass/volume ratio”? 7. Check the values of the “mass to volume ratios” for distilled water, salt water, wood, aluminum, iron, and lead and compare those values to the ones you calculated in your chart above. How did you do? Suggest a possible reason for your numbers to not be exactly the same as the published values. 8. Create a simple line graph using the origin (0,0) as the first point for each solid, and then plot the (mass, volume) for each solid and connect those two points. Try to fit all the solids on one graph, but it if it becomes too crowded, do more than one graph using the same scale for your axes. Conclusion: “What you did” and “what you found”. Be sure to address BOTH of the purposes !