Mystery Cube Density Lab
advertisement

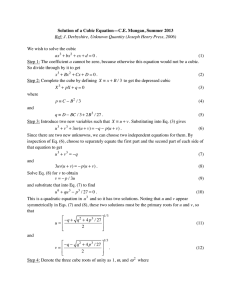
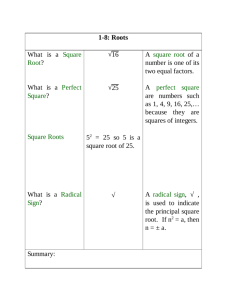
Does It Sink or Float??? Water Density Experiment Fremont PDMS 19 and 20 of February 2014 What is density? The density of a substance is the relationship between the mass of the substance and how much space it takes up (volume). Density = mass/volume Mass usually expressed in grams Volume usually expressed in cubic centimeter Density of Water Water molecules have the same mass and size. Therefore, density is the same for any amount of water: 1 gram per cubic centimeter Objects Verses Water Because the density of water equals 1 g/cubic cm: Any object with a density above 1 g/cubic cm will sink Any object with a density below 1 g/cubic cm will float Objective of Experiment Students will be able to measure the volume and mass of water and calculate its density. Students will be able to explain that since any volume of water has the same density, at a given temperature, that density is a characteristic of water. Materials Density Cub Set Ruler Balance Your handy dandy science NOTEBOOK! Procedures (1) Find the mass of each cube using a balance and record results in the data table. (2) Find the volume of each cube (Length x Width x Height) and record results in the data table. (3) Calculate the density and record the results in the data table: Density = mass/volume Results Cube # Mass (g) Volume (cm^3) Density (g/cm^3) Conclusion What determines whether a cube will float or sink in water? Which cubes would sink in water? Which cubes would float in water? Describe how you determined the volume of the cubes. Describe how you found the density for each cube. BONUS! Ask Ms. Kelley, Ms. Herrera, or Ms. Mels if you and your group can test your conclusion! This must be done once you have completed your result and conclusion section.