Analytical Techniques
advertisement

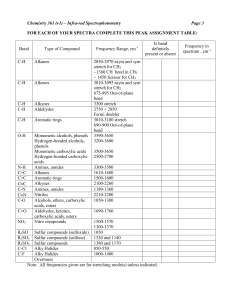
INFRARED SPECTROSCOPY THEORY A molecule is not a rigid assemblage of atoms. The atoms, even in the solid state, vibrate about an equilibrium position. Each atom vibrates with a frequency which depends on its mass and the length and strength of any bonds which it has formed. There are two kinds of fundamental vibrations for molecules, stretching and bending (or deformation). Stretching In stretching vibrations, the distance between the atoms increases or decreases, but the atoms remain in the same bond axis. symmetrical stretching asymmetrical stretching Bending In bending vibrations, the position of the atom changes relative to the original bond axis. + + + - scissoring rocking wagging twisting (in-plane bending vibrations) (out-of-plane bending vibrations) + and – signify vibrations above and below the plane of the paper respectively Each of the various stretching and bending vibrations of a bond occur at a certain particular frequency, in the range 1.20 x 1013 to 1.20 x 1014 Hz, which corresponds to a frequency in the infrared region of the electromagnetic spectrum. Therefore, when infrared radiation of that same frequency falls on the molecule, energy is absorbed and the amplitude of that vibration increases. Only those vibrations which cause a change in the dipole moment of the molecule will result in the absorption of infrared radiation. So that the numbers involved are more convenient, absorption is usually quoted at a particular wavenumber (the reciprocal of the wavelength in cm of the absorbed radiation), which has the unit cm-1. Bending vibrations generally require less energy and therefore occur at lower wavenumbers than stretching vibrations. Stretching vibrations occur in the order of bond strengths. Thus, a triple bond absorbs radiation of a higher wavenumber than the equivalent double bond, which in turn absorbs radiation of a higher wavenumber than the equivalent single bond. TOPIC 13.15: ANALYTICAL TECHNIQUES 1 THE INFRARED SPECTROMETER An infrared spectrometer has two beams of radiation from the same source, one passing through the sample, the other passing through a reference cell. Sample Chart recorder Detector glowing source Reference Monochromatic grating A rotating segmented mirror takes information from the sample and reference beams alternately. If a particular frequency is absorbed by the sample, less radiation is transmitted, and the detector compares the intensity of radiation of each wavelength passing through the sample with the intensity passing through the reference cell. When the spectrum is determined, a calibration line is usually recorded on the paper. For this purpose, the absorption peak of polystyrene at 1603cm -1 is commonly used. Preparing Samples Glass absorbs infrared radiation. Therefore, cells for use in an infrared spectrometer have to be made from some other material, which is transparent to infrared. Sodium chloride and potassium bromide are frequently used. Potassium bromide is preferred, because sodium chloride absorbs radiation below 625cm-1. Gases Gases are placed in a special cell, typically 10cm in length. Liquids Liquids are examined in the form of a thin film, formed by sandwiching a small drop of liquid between sodium chloride discs. Solutions Solutions, usually in solvents such as CCl4 and CHCl3, are placed in cells with a path length between 0.01mm and 0.5mm. Solids A disc can be made by finely grinding the solid with KBr in a mortar. The powder is then placed in a circular die and compressed to give a transparent disc which is used to record the spectrum. Alternatively, a paste can be made from the solid and a long-chain liquid hydrocarbon called Nujol. This mull is then placed between sodium chloride discs. TOPIC 13.15: ANALYTICAL TECHNIQUES 2 INTERPRETATION OF INFRARED SPECTRA Infrared spectra are valuable aids in the identification of unknown compounds. A complex molecule has a large number of vibrational modes. Some of these vibrations (mainly above 1500cm-1) are associated with individual bonds or functional groups, while others (below 1500cm-1) are considered as vibrations of the whole molecule. The absorption associated with a particular individual bond or functional group always occurs at approximately the same wavenumber, irrespective of the molecule involved. e.g. the carbonyl (C=O) absorption in the following aliphatic ketones: butanone 1718cm-1 cyclohexanone 1715cm-1 Structural changes close to the vibrating bond can cause small shifts. e.g. the carbonyl absorption in the following classes of compounds: aliphatic ketones 1725-1705cm-1 aromatic ketones 1700-1680cm-1 aliphatic aldehydes 1740-1720cm-1 aromatic aldehydes 1715-1695cm-1 esters 1750-1735cm-1 aliphatic carboxylic acids 1725-1700cm-1 acyl chlorides 1795cm-1 The infrared spectrum can conveniently be split into four regions for interpretation. 4000-2500 cm-1 absorption due to stretching vibrations of single bonds to hydrogen e.g. C-H, O-H, N-H 2500-2000 cm-1 absorption due to stretching vibrations of triple bonds e.g. C=C, C=N 2000-1500 cm-1 absorption due to stretching vibrations of double bonds e.g. C=C, C=O 1500-400 cm-1 absorption due to stretching vibrations of some single bonds e.g. O-H, C-O absorption due to various bending vibrations The region 1500-400 cm-1 is not mainly used for recognising individual bond absorptions. This region contains absorption bands which are characteristic of the compounds under test and no other compound. This region is therefore called the fingerprint region. The fingerprint region can be used to confirm the identity of a substance by comparison with the infrared spectrum of an authentic sample. TOPIC 13.15: ANALYTICAL TECHNIQUES 3 Correlation Table for Infrared Spectroscopy 4600 4200 3800 aliphatic C-H stretch 3400 3000 2600 2200 2000 1800 N-H stretch amines (v) 1600 1400 1200 1000 C-N stretch C-H bend aromatic nitro cpds. aliphatic (v) C-H stretch C C-H (sh) C N stretch aliphatic nitriles C=O stretch (vs) C-H stretch alkenes (sh) C C-H stretch aromatic ring (w-m) C stretch (v) C-Br stretch haloalkanes 2600 C=C stretch Ar-C=C (w-m) Abbreviations used: w = weak m = medium s = strong vs = very strong v = variable br = broad sh = sharp C-N stretch aromatic nitro cpds. N-H bend (v) 2200 2000 1800 1600 1400 Wavenumber / cm-1 TOPIC 13.15: ANALYTICAL TECHNIQUES 4 C-Cl stretch haloalkanes C=C stretch aromatic (m) C-H stretch (2 bands) aldehydes 3000 C-N bend amines C=C stretch alkenes (v) C-H stretch alkanes (v) 3400 C-H bend C=C-H (m-s) C-C skeletal vibration (w) O-H stretch ROH (sh) 3800 C-H bend C C-H (s) N-O stretch aromatic nitro cpds. H-bonded O-H stretch (br) 4200 400 C-O stretch esters, alcohols, carboxylic acids O-H stretch COOH (w, br) 4600 600 C-O-C asymmetric stretch (vs) free O-H stretch (sh) aromatic C-H stretch (w) 800 Fingerprint region 1200 1000 800 600 400 Characteristic Infrared Absorption Ranges Wavenumber range/ cm-1 Functional Group Vibration 3750 – 3200 3500 – 3300 3500 – 3140 3300 – 2500 3095 – 3010 3080 – 3030 2962 – 2853 2900 – 2700 (2 bands) 2260 – 2215 2260 – 2140 1850 – 1800 1790 – 1740 1795 1750 – 1735 1740 – 1720 1730 – 1717 1725 – 1705 1725 – 1700 1700 – 1680 1700 – 1630 1669 – 1645 1650 – 1590 1600, 1500 & 1450 1570 – 1500 1550 – 1330 1485 – 1365 1370 – 1300 1310 – 1100 880 – 700 800 – 600 600 – 500 alcohol & phenol amine amide carboxylic acid alkene arene alkane aldehyde nitrile alkyne carboxylic anhydrides carboxylic anhydrides acyl chloride ester aldehyde aryl ester ketone carboxylic acid aryl carboxylic acid amide alkene amide & amine arene aromatic nitro compound aromatic nitro compound alkane aromatic nitro compound ester, alcohol, carboxylic acid arene chloroalkane bromoalkane O-H stretch N-H stretch N-H stretch O-H stretch C-H stretch C-H stretch C-H stretch C-H stretch C=N stretch C=C stretch C=O stretch C=O stretch C=O stretch C=O stretch C=O stretch C=O stretch C=O stretch C=O stretch C=O stretch C=O stretch C=C stretch N-H bend C=C stretch C-N stretch N-O stretch C-H bend C-N stretch C-O stretch C-H bend C-Cl stretch C-Br stretch TOPIC 13.15: ANALYTICAL TECHNIQUES 5 MASS SPECTROMETRY Mass spectrometry is a useful technique for determining the structure of a compound. It is about 1000 times more sensitive than either infrared or NMR spectroscopy and requires only a microgram or less of the sample. The basics of this topic were covered in module CHM1. BASIC FEATURES 1. A substance is vaporised. For gases and volatile liquids this is done simply by injecting them into the ionisation chamber, which is at a very low pressure. Less volatile liquids and solids are introduced into the mass spectrometer through a heated inlet. 2. The molecules in the vapour are bombarded in the ionisation chamber with a beam of high energy electrons. This results in: a) the formation of positively charged molecular ions b) the breaking of chemical bonds and the formation of a range of lighter fragment ions 3. The ions are accelerated by an electrostatic field and collimated into a narrow beam. In practice, the ions generated in 2) have a range of kinetic energies. If the path through the electrostatic field is curved, the ions are deflected according to their kinetic energies, and only those of a certain energy can pass through the exit slit. This improves the resolution of the instrument. These double resolution instruments can detect mass differences of 1 in 10 6. 4. The ions are passed through a magnetic field. Here the ions are separated according to their mass:charge ratio (m/z). In practice most ions carry a single charge. For a given charge, those ions with the lightest mass are deflected most. vaporised sample (+) ionisation chamber electron gun (+) accelerating electric field (-) heavier ions lighter ions recorder magnetic field ions of intermediate mass amplifier ion detector to vacuum pump 5. The separated streams of ions are focused in turn on to a detecting device by varying the magnetic field. This leads finally to a plot of the signal from the recorder against m/z being drawn out on a pen recorder or recorded photographically. TOPIC 13.15: ANALYTICAL TECHNIQUES 6 The mass spectrum consists of a series of sharp peaks; the abundance of an ion is proportional to its peak height. It is usual to convert the spectrum to a stick diagram in which only the major peaks are drawn, the height of the line representing a percentage of the height of the highest peak. e.g. stick diagram of the mass spectrum of ethanol. Relative abundance % [CH2OH]+ 100 80 60 parent ion m/e 46 [C2H5O]+ 40 [C2H5]+ [C2H3]+ [C2H5OH]+ 20 [M+1]+ 10 20 30 40 50 m/z Applications of Mass Spectrometry 1. Mass spectrometry can be used for the determination of the isotopic composition of a sample of an element and hence for the measurement of relative atomic mass. 2. 3. A mass spectrum can be used to determine relative molecular masses of compounds since the line with the highest m/z value is usually the molecular ion, M+. There is always a very small peak at (M+1) for organic compounds which is due to the 13C isotope, present with a natural abundance of 1.1%. 4. The structure of a molecule can be determined from the fragmentation pattern. Once the molecular ion has been formed, it will usually decompose into smaller fragments. By looking at the major peaks in the mass spectrum, it is possible to decide which fragments have been lost and thence to build up a picture of the original molecule. Occasionally a rearrangement of an ion may take place. TOPIC 13.15: ANALYTICAL TECHNIQUES 7 Common Losses from Molecular Ions Ion M-15 M-16 M-17 M-18 M-27 M-28 M-29 M-30 M-31 M-32 M-36 M-41 M-42 Group being lost CH3 O NH2 OH H2O HCN CO C2H4 CHO C2H5 OCH2 NO OCH3 CH3OH HCl C3H5 CH2CO C3H6 M-44 M-45 M-46 CO2 COOH C2H5OH NO2 Possible inference -NO2 -CONH2 alcohol alcohol, aldehyde, ketone nitrile quinone aromatic ethyl ether (ArOC2H5) ethyl ester (RCOOC2H5) propyl ketone (RCOC3H7) aldehyde ethyl ketone (RCOC2H5) aromatic methyl ether (ArOCH3) aromatic nitro compound methyl ester (RCOOCH3) methyl ester (RCOOCH3) primary haloalkane propyl ester (RCOOC3H7) methyl ketone (RCOCH3) aromatic ethanoate (CH3COOAr) butyl ketone (RCOC4H9) aromatic propyl ether (ArOC3H7) carboxylic acid, ester, acid anhydride carboxylic acid ethyl ester (RCOOC2H5) aromatic nitro compound Aromatic compounds often produce fragment ions at m/z 77 (C6H5+) and m/z 91 (C6H5CH2+). TOPIC 13.15: ANALYTICAL TECHNIQUES 8 Interpretation of the Mass Spectrum If the likely structure of the compound is known, it can usually be confirmed by considering the fragmentation pattern. It is not always possible to distinguish between isomers e.g. the different disubstituted benzenes. If the structure is unknown, the mass spectrum can be compared with libraries of mass spectra which are commercially available on computer software. The mass spectra of all compounds having the same Mr (or molecular formula) as the unknown can be called up and compared. A compound containing one atom of chlorine will give two molecular ions which are two units apart. The higher m/z peak will have an intensity one third that of the lower m/z peak. This is because chlorine contains the isotopes 35Cl and 37Cl in the ratio 3:1. A compound containing one atom of bromine will give two molecular ions which are two units apart. Both peaks will be equal in intensity. This is because bromine contains two isotopes 79Br and 81Br in the ratio 1:1. High-Resolution Mass Spectrometry In the Atomic Structure topic, we learned that a mass spectrometer can be used to work out isotopic abundances and hence relative atomic masses. It was also noted that the m/z value of the molecular ion peak (the peak with the highest m/z value) can give the Mr of a compound. A more sensitive high-resolution mass spectrometer can measure the mass of a molecular ion to several decimal places. Using very precise relative atomic masses, molecules with similar relative molecular masses can be distinguished from each other. Element Hydrogen Carbon Nitrogen Oxygen Chlorine Symbol H C N O Cl Relative atomic mass Ar 1 decimal place 4 decimal places 1.0 1.0079 12.0 12.0107 14.0 14.0067 16.0 15.9994 35.5 35.4532 For example, methanal and ethane have the same Mr values when determined via in low resolution mass spectrometry: H2C=O Mr = (12 x 1) + (1 x 2) + (16 x 1) = 30.0 C2H6 Mr = (12 x 2) + (1 x 6) TOPIC 13.15: ANALYTICAL TECHNIQUES 9 = 30.0 However, using a more sensitive high resolution mass spectrometer a difference is observed: H2C=O Mr = (12.0107x1) + (1.0079x2) + (15.9994x1) = 30.0259 C2H6 Mr = (12.0107x2) + (1.0079x6) = 30.0688 The difference is very small but is enough to uniquely identify each compound. The high resolution mass spectrometer enables very precise measurements of masses to be made. Since the mass defect varies from element to element, the masses of ions with the same nominal mass show small differences if precise measurements are made. E.g. for a nominal m/z of 28: Ion Precise mass CO+ N2+ CH2N+ C2H4+ 27.994914 28.006148 28.018724 28.031300 Thus it is possible with a high resolution spectrum to determine not only the molecular mass but also the molecular formula. High-Resolution Mass Spectrometry – Example Questions Q1. The molecular ion peak in a high-resolution mass spectrum has an m/z ratio of 58.0789. The substance is either butane or propanone. Calculate the exact molecular mass of both substances to 4 d.p. and then deduce which it is. ………………………………………………………………………………………………. ………………………………………………………………………………………………. ………………………………………………………………………………………………. ………………………………………………………………………………………………. ………………………………………………………………………………………………. ………………………………………………………………………………………………. TOPIC 13.15: ANALYTICAL TECHNIQUES 10 Q2. An unknown substance (X) was put through a high resolution mass spectrometer. The resulting molecular ion peak had a value of m/z = 60.0518 to 4 d.p.. An infrared absorption spectrum showed an intense absorption at 1740cm-1 and a broad absorption at 2500-3000cm-1. The substance was then heated with acidified potassium dichromate and no colour change was noted. Deduce the identity of X and explain your answer. ………………………………………………………………………………………………. ………………………………………………………………………………………………. ………………………………………………………………………………………………. ………………………………………………………………………………………………. ………………………………………………………………………………………………. ………………………………………………………………………………………………. Q3. Unlabelled samples of glucose (C6H12O6) and aspirin (C9H8O4) were mixed up. Explain and show how high resolution mass spectrometry could distinguish between the two. ………………………………………………………………………………………………. ………………………………………………………………………………………………. ………………………………………………………………………………………………. ………………………………………………………………………………………………. ………………………………………………………………………………………………. ………………………………………………………………………………………………. TOPIC 13.15: ANALYTICAL TECHNIQUES 11