Molecular Geometry & VSEPR Theory
advertisement

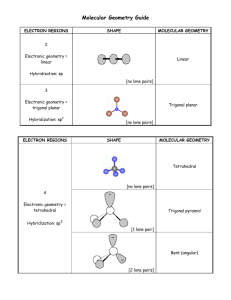
MOLECULAR GEOMETRY How can molecular geometry (shape) be predicted ? VSEPR THEORY Valence Shell Electron Pair Repulsion Theory The electron pairs (both bonding and non-bonding) surrounding a central atom are positioned as far as possible form each other, thus minimizing electron-pair repulsions. Number of Pairs Arrangement of Pairs Geometry 2 Linear 3 Trigonal Planar 4 Tetrahedral 6 Number of Pairs Arrangement of Pairs Geometry 5 Trigonal bipyramidal 6 Octahedral 7 Lewis formula Electron Pairs Total Bonding Lone Electron Pair Geometry Molecular Geometry Example AX2 X:A:X 2 2 0 FBeF Linear Linear Bond Angle = 1800 O=C=O Nonpolar Treat double bonds as single bonds AX3 F X .. A:X .. X B 3 3 0 Trigonal planar 8 Bond Angle = 1200 Nonpolar Trigonal Planar F F Lewis formula AX2E E = lone pair .. X : A :: X or .. X :: A : X Treat double bonds as single bonds AX4 X .. X:A:X .. X Electron Pairs Total Bonding Lone Electron Pair Geometry Molecular Geometry Example S 3 2 1 O Trigonal planar O Bent (angular) Bond Angle 1200 Polar Cl C 4 4 0 Cl Tetrahedral 9 Tetrahedral Bond Angle 109.50 Nonpolar Cl Cl Lewis formula AX3E E = lone pair .. X:A:X .. X Electron Pairs Total Bonding Lone 4 3 Electron Pair Geometry Example 1 N H Tetrahedral AX2E2 .. X:A: .. X Molecular Geometry H H Trigonal Pyramidal Bond Angle 109.50 Polar O 4 2 2 H Tetrahedral 10 Bent (Angular) Bond Angle 109.50 Polar H Lewis formula Electron Pairs Total Bonding Lone Electron Pair Geometry Molecular Geometry Example AX5 Cl X X Cl X A Cl 5 5 P 0 Cl Cl X X Trigonal bipyramidal Trigonal bipyramidal Bond Angles: 1200 (3) and 900 (6) Nonpolar PCl5 SF4 AX4E F F X .. X:A:X .. X S F 5 4 1 Trigonal bipyramidal 11 Seesaw Bond Angles: 1200, 900, and 1800 Polar F Lewis formula Electron Pairs Total Bonding Lone Electron Pair Geometry Molecular Geometry Example AX3E2 X .. X:A .. X ClF3 F F 5 3 2 Trigonal bipyramidal T-shaped Bond Angles: 900 Polar AX2E3 X .. A .. X Cl F XeF2 F 5 2 3 Xe Trigonal bipyramidal 12 Linear Bond Angle = 1800 Nonpolar F Lewis formula Electron Pairs Total Bonding Lone Electron Pair Geometry Molecular Geometry Example AX6 SF6 X X F X A X F 6 6 F 0 S X F X Octahedral F Octahedral Bond Angle = 900 Nonpolar AX5E F IF5 F X X F X 6 5 1 I A X F X F Octahedral 13 Square pyramidal Bond Angle: 900 Polar F Lewis formula Electron Pairs Total Bonding Lone Electron Pair Geometry Molecular Geometry Example AX4E2 X X A X XeF4 F 6 X 4 2 F Xe Octahedral 14 Square planar Bond Angle: 900 Nonpolar F F SUMMARY Symmetrical Arrangement Asymmetrical Arrangement Polar Bonds Nonpolar Molecule Polar Bonds Polar Molecule Linear Bent Trigonal Planar planar Trigonal pyramidal Tetrahedral Seesaw Trigonal bipyramidal T-shaped Octahedral Square pyramidal Square planar 15 BOND ANGLES Can be approximately predicted from the VSEPR model. Some deviations from the predicted bond angles have been determined experimentally. These deviations are caused by 2 factors: 1. Effect of Lone Pairs A lone pair tends to require more space than a bonding pair Reason: A lone pair of electrons is attracted to only one atomic core, whereas a bonding pair is attracted to two. The lone pair is larger, while the bonding pair is drawn more tightly to the nuclei. Lone pair Bonding Pair Lone pairs repel each other stronger than Bonding Pairs. Result: The repulsions between electron pairs depend on the type of electron pairs involved. REPULSION INCREASES Bonding Pairs Bonding Pairs Bonding Pairs Weakest Repulsion Lone Pairs Lone Pairs Lone Pairs Strongest Repulsion 16 CH4 Electron Pair Geometry Expected Bond Angle Tetrahedral NH3 Tetrahedral H2O Tetrahedral 109.50 109.50 109.50 Bonding Pairs 4 3 2 Lone Pairs 0 1 2 Actual Bond Angle 109.50 1070 17 1050 2. Effect of Multiple Bonds Muiltiple bonds require more space than single bonds because of the greater number of electrons: Electron Pair Geometry: Trigonal Planar Expected Bond Angle: 1200 Actual Bond Angle: 1160 Reason: C = O CH Stronger Repulsion CH CH Weaker Repulsion 18 Sample Problems I. Predict the geometry of the SCl2 molecule. Is the molecule polar ? Step 1 :Write the electron-dot formula. 1 S = 1 x 6 electrons = 6 electrons 2 Cl = 2 x 7 electrons = 14 electrons Total: 20 electrons .. .. .. : Cl : S : Cl : .. .. .. Step 2: Step 3: Determine how many electrons are there around the central atom ? 4 How many bonding pairs? 2 How many lone pairs? 2 Type of molecule ? AX2E2 Step 3: Detemine the electron pair geometry for 4 electron pairs Tetrahedral Step 4: Sketch the molecule surrounded by both bonding and nonbonding electron pairs 19 Step 5: Obtain the molecular geometry from the directions of the bonding pairs. Sketch the molecule again, but ignore the lone pairs. Answer: The molecule has a bent (angular) gemetry and it is POLAR. II. Predict the geometry of the COCl2 molecule. 1 C = 1 x 4 electrons = 4 electrons 1 O = 1 x 6 electrons = 6 electrons 2 Cl = 2 x 7 electrons= 14 electrons Total: 24 electrons :O: .. .. O .. .. : Cl : C : Cl : .. .. .. : Cl .. C .. Cl : .. Note: Count a double bond as one pair (one group of electrons) Number of electron pairs? Number of bonding pairs? Number of lone pairs ? 3 3 0 20 Note: When there are no lone pairs, the Electron Pair Geometry and the Molecular Geometry are the same. The Geometry for 3 electron pairs is Trigonal Planar O C Cl 21 Cl