CEE 437 Minerals “Lab”
advertisement

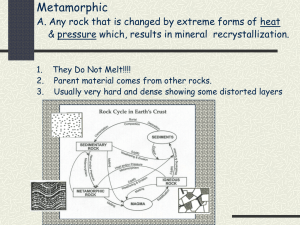
CEE 437 Minerals “Lab” Earth Materials That are Not Minerals Some earth materials are not minerals as they lack a crystalline structure. Here are some examples: Chert/Flint: Like the hydrous form, opal, this is amorphous (i.e. non crystalline) silica. Cherts are generally forming by dissolution from groundwater. Often this takes the form of secondary replacement in sediments and sedimentary rocks. A common occurrence is in the form of nodules or layers that range from a few cm to meter or so scales. The other major form of silica (not shown here) is organic silica which is formed by sedimentary processes from the microscopic silica shells of diatoms. This flint was found in a recently exposed n Denmark. It has been broken possibly by human activity (note conchoidal fracture surfaces). This was a very significant material for stone-age human exploitation. Glass: This is actually an artificial glass, made by in situ melting by a company called “Geosafe”. Their idea was to make subsurface barriers to ground water flow, i.e. for contaminant isolation, by melting the soil or rock in place with buried heaters. This is an example of the resulting material. Volcanic glasses are not much different in appearance. SHARP SURFACES!! Be careful to avoid cutting yourself Coal: Coal is one type of organic material that forms rocks but is not a mineral. This coal is from the Newcastle coal field in suburban Seattle. Pumice: A frothy volcanic glass. Source: Mt. St. Helens. Basics of Mineralogy The collection of mineral specimens here shows the range of crystal forms and habits. This group illustrates cleavage, luster, hardness, crystal habit and other features useful for mineral identification. Included is selenite (or gypsum). Very soft (<fingernail). Composition calcium sulfate, and a precipitate from seawater into beds (source for plaster), or void fills precipitated from groundwater. This is nice specimen so be discrete in scratching. Also here is calcite CaCO3, the main component of limestone, also occurring as sedimentary beds or precipitated from groundwater. Readily identified by hardness and fizzing in weak HCl acid. Can be a very minor component in igneous rocks (Africa has a really weird bicarbonate of soda volcano). Calcite is very important component of sedimentary cements. A common variant is dolomite (after Dolomite Alps in Italy) which substitutes Mg for half the Ca. Identical crystal form, but not attacked by weak HCl acid. Note “dogtooth spar” crystal habit. Cleaves into rhombs (try this on smaller samples). A core sample of rock salt. Try the taste test if you trust your fellow student’s hygiene. This is a few large crystals, though cur circularly by the coring. Note brine inclusions. Another sample of an evaporite is the red rock with clear cubes. Any guess what the cubes are. The red material (carnallite) is a salt formed from Mg and K. It is considerably more soluble than normal NaCl. K-salts like these are the main source of potash for fertilizers. Rock salt, anhydrite, and calcite are all soluble to different degrees. Consider the engineering problems that might arise when rock containing large amounts of these materials exists near the surface. Purple sample of fluorite (CaF2). Classic cubic crystal form but cleave into octahedral (note inside crystals). Contrast with Galena (PbS) which is silvery metallic and grows as cubes and cleaves as cubes. Pyrite (FeS2). Three samples. Large cube, and a smaller sample in dodecahedral habit. Note also sample where pyrite has replaced original material in a scallop shell. Pyrite is common associate of ore minerals also appearing in plutonic rocks as well as in sediments that are in chemically reducing conditions (lack of oxygen means Fe ties with S instead of O). Sphalerite (ZnS): Massive chunk of ore from Balmat Mine, NY. Note cleavage surfaces and sub-metallic luster. Rock-Forming Minerals There is an assortment of rock forming mineral specimens shown here. Note particularly the quartz (hexagonal prisms, pyramidal terminations). One only gets nice crystal forms like this where there is a void in the rock for the crystals to grow into or where the medium is very soft (clay, mica) compared to the crystal. Also note olivine (green aggregate of small crystals, also know in gem form as peridot). A mantle rock also has this name, peridotite. Two feldspar samples are here. The whitish one is K-or orthoclase feldspar, associated with the acid or felsic or hi-Si end of the igneous spectrum, and the other is plagioclase (actually a cut specimen but notice the banding from crystal twinning. The K-feldspar is from a weathered pegmatite in California. Amphibole is present in the sample of “Amphobolite”. Use the hand lens to see small lathes of elongated dark crystals. This with pyroxene is the main ferro-magnesian mineral families common to silicate rocks. This amphibolite is from a hydroelectric power tunnel in Quebec. The dark green, greasy sample is serpentine or serpentinite. It is formed by hydrothermal alteration of peridotites, and how one usually finds sub-crustal rocks when they have been sliced up into the crust. This sample is from Ruby Creek, Blewett Pass. It will be one of our field trip stops. Quartz is the “glassy” looking mineral occurring well-formed crystals. What is the symmetry group of this mineral? What can you say about its cleavage properties? What in the crystal structure would lead to this condition? The dark brown crystal is garnet. A cubic mineral, here in a dodecahedral habit. Can e a gem when clear. Also, a common, though minor rock-forming constituent. Can be a good indicator of temperature and pressure conditions in metamorphic rocks. Putting it together into rocks The selection of samples shows how minerals aggregate into rocks. Start looking at the pegmatite. Pegmatite is a form of granite, a late stage melt in the freezing of a magma body. As a late stage melt, it concentrated many odd components as well as water, often forming spectacular mineral specimens. This sample (slightly weathered) shows the basic granite minerals in a very course form, quite visible to the naked eye. Note the white mica (muscovite), quartz (glassy appearance, no cleavage) and orthoclase feldspar (milky, clear cleavage). Make a sketch showing the mineral component. Note if there any evidence of weathering on this sample. After looking at the pegmatite take the sample of granite. Granite has the same mineral components (perhaps with black mica, biotite, and amphibole) as a FeMg component. Granite from by freezing from magma (melts). For comparison note the sample of gneiss. It looks a lot like the granite, but it rather than having a uniform “granular” texture/fabric, it is strongly layered or “foliated” (after folia, or leaves in Latin). The fabric reveals the metamorphic origin of this rock, formed by solid-state recrystallization under temperature and pressure. Also note here two samples of schist. One is Manhattan schist, the bedrock of New York City, and is a muscovite-dominated mica schist likely derived from continental, High Si material, like shales, or sandstones. The other is from Icicle Creek in near Leavenworth in the Cascades near Hwy 2. This rock is a biotite schist with clear garnet inclusions (porphyroblasts). How does foliation affect the mechanical properties of the rock? How do these properties relate to the crystal structure of the mineral components? Two specimens of volcanic rock (one pebble, other a cut bowl). Note “phenocrysts”, or crystals of feldspar that were already formed when this was blown out of a volcano. The rapid cooling preserves the crystals but quickly chills the rest into a glass or finely crystallized ground mass. The texture contrast with granite differentiates the volcanic versus plutonic origin from melt. Finally, note a sample of claystone. This has no visible crystalline material in it, but the analyses indicate that is about equal thirds of very fine grained clay minerals, quartz, and calcite (both as cements and as sediment grains). This sample is from a depth of 400 m in a corehole in France. What might happen if it is immersed in fresh water? Note where each rock specimen might be formed on the figures below: