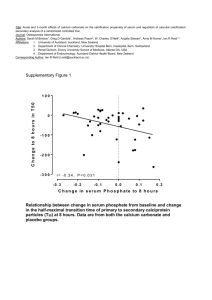
Precipitated calcium carbonate (PCC) is an extremely versatile filler
advertisement

Dr. sc. Damir Kralj Laboratory for Precipitation Processes Division of Material Chemistry Rudjer Boskovic Institute, Zagreb 07.3. An outline of the Project proposal: DEVELOPMENT OF AN ADAPTABLE PROCEDURE FOR THE PREPARATION OF PRECIPITATED CALCIUM CARBONATE (PCC) OBJECTIVES Calcium carbonate is a slightly soluble salt of carbonic acid that appears as a wide spread mineral in the nature and also as a product, or a component of a product, on the market. Thus for instance, calcium carbonate is a versatile filler and pigment that is utilized in paper, plastics, rubber, paint, textiles, pharmaceuticals and food. Therefore, the demands for its physical chemical properties are conditioned by the way of application. The natural ground calcium carbonate (GCC), obtained by mechanical treatment of the natural materials (limestone, marble, chalk and coral), does not often meet market demands for high quality of a product. In order to satisfy these needs, special calcium carbonate fillers and pigments must be used. A wide variety of calcium carbonate particle shapes and sizes, as well as size distributions, can be produced via precipitation processes. The precipitated calcium carbonate (PCC), compared with GCC, has generally more uniform particle size distribution of finer particle sizes and a higher degree of chemical purity. In addition, calcium carbonate can precipitate in six solid modifications: three hydrated forms (amorphous calcium carbonate, calcium carbonate hexahydrate, calcium carbonate monohydrate) and three anhydrous polymorphs (calcite, aragonite and vaterite). Natural GCC is mostly calcite. Each of the possible precipitating crystalline modifications of calcium carbonate crystallizes in different crystal forms, thus enabling the production of a variety of crystal shapes. The mostly used technological process of PCC production is precipitation, initiated by the recarbonation of milk of lime. Precipitation, i.e. the crystallization of sparingly soluble substances, is one of the most important operations in chemical, pharmaceutical and many other process industries. Precipitation may be defined as a formation of a new, solid, phase in a homogeneous solution. The process starts with the formation of nuclei in a supersaturated solution (homogeneous or heterogeneous nucleation), continues by growing nuclei into crystallites and terminates by aging of solid particles to a size that causes their sedimentation. The principal parameters that determine the physical chemical properties of precipitate are the initial supersaturation (concentration of reactants), the temperature and the presence of dissolved or suspended impurities. Control of the properties in a technological process of PCC production is still mostly based on the experience rather than the numerous scientific studies of kinetics and mechanisms of the calcium carbonate precipitation. The knowledge and experience that have been colected in the Laboratory for Precipitation Processes during the years of detailed fundamental studies of the kinetics and mechanisms of precipitation processes and particulary of calcium carbonate polymorphs (see the list of the selected papers) supports our belief that we are able to develop a procedure applicable for an industrial production of PCC. In this procedure, by using kinetic, thermodynamic and hydrodynamic factors and changing process parameters, the physical chemical properties of precipitate (mineralogical composition, particle size distribution and morphology) can be controlled. Lime and carbon dioxide are considered as the basic precipitation components. The initial investigations will be held in the laboratory scale equipment using analytical grade chemicals. The later stage will involve the scale up of the laboratory equipment and the use of limestone and technical grade chemicals. The efforts will be made to automatize the process and, if possible, to develop a continuous production. STRENGTHS AND OPPORTUNITIES Experience and an international recognition of the proposer and other investigators of the Project in the field of precipitation processes, particularly in the precipitation of calcium carbonates; Suitable laboratory equipment necessary for proposed investigations; Limestone, as the basic raw material in the PCC production; such material found in Croatia is of high quality and readily available; In spite of the need of market and the variety of advantages of the product, there is no production of PCC in Croatia so far; Declarative support of 5 Croatian companies (quicklime manufacturers, paper industry) to the Project; The installed capacities of the Croatian companies for quicklime production, which is the raw material for the PCC production, exceed the necessities at the market; All necessary infrastructure for a new plant allready exists at locations of the existing plants for quicklime production; The process of PCC production is environment friendly and does not emanate toxic pollutants. In addition, carbon dioxide (CO2), the by-product of limestone calcination, can be used for carbonation of hydrated lime slurry thus minimazing the emmision of this "green house" gas in the atmosphere. Global market demands for high purity (quality) PCC is increasing in pharmaceuticals and the plastics industry (growth rates up to 7% pa over next 5 years); The know-how significantly contributes to the price structure of PCC and therefore, the knowledge could become a product in itself, interesting on the global market (patent). WEAKNESSES Lack of experience in the scale up from the laboratory, or bench, scale to the pilot plant production; Economical weakness of the Croatian companies result in their reluctance to support the initial stage of the Project realization financially. Laboratory for Precipitation Processes Division of Material Chemistry The list of publications on calcium carbonate precipitation 1. D. Kralj, Lj. Brečević and A.E. Nielsen: Vaterite Growth and Dissolution in Aqueous Solutions. I. Kinetics of Crystal Growth. Journal of Crystal Growth 104 (1990) 793800. 2. F.A. Andersen and D. Kralj: Determination of the Composition of Calcite-Vaterite Mixtures by Infrared Spectrophotometry. Applied Spectroscopy 45 (1991) 1748-1751. 3. D. Kralj, Lj. Brečević and A.E. Nielsen: Vaterite Growth and Dissolution in Aqueous Solutions. II. Kinetics of Dissolution. Journal of Crystal Growth 143 (1994) 269-276. 4. D. Kralj and Lj. Brečević: Dissolution Kinetics and Solubility of Calcium Carbonate Monohydrate. Colloids and Surfaces 96 (1995) 287-293. 5. Lj. Brečević, V. Nöthig-Laszlo, D. Kralj and S. Popović: The Effect of Divalent Cations on the Formation and Structure of Calcium Carbonate Polymorphs. Journal of Chemical Society, Faraday Transaction 92(6) (1996) 1017-1022. 6. J. Perić, M. Vučak, R. Krstulović, Lj. Brečević and D. Kralj: Phase Transformation of Calcium Carbonate Polymorphs. Thermochimica Acta, 277 (1996) 175-186. 7. D. Kralj, Lj. Brečević and J. Kontrec: Vaterite Growth and Dissolution in Aqueous Solutions. III. Kinetics of Transformation. Journal of Crystal Growth, 177 (1997) 248257. 8. D. Kralj and N. Vdović: The Influence of Some Naturally Occurring Minerals on the Precipitation of Calcium Carbonate Polymorphs. Water Research 34(1) (2000) 179-184. 9. N. Vdović and D Kralj: Electrokinetic properties of spontaneously precipitated calcium carbonate polymorphs: the inflluence of organic substances. Colloids and Surfaces A: 161 (2000) 499-505. 10. F.A. Andersen and Lj. Brečević: Infrared Spectra of Amorphous and Crystalline Calcium Carbonate. Acta Chemica Scandinavica 45 (1991) 1018-1024. 11. Lj. Brečević and A.E. Nielsen: Solubility of Calcium Carbonate Hexahydrate. Acta Chemica Scandinavica 47 (1993) 668-673. 12. N. Kallay, V. Tomašić, S. Žalac and Lj. Brečević: Calorimetric Investigation of Kinetics of Solid Phase Dissolution: Calcium Carbonate Dissolution in Aqueous EDTA Solution. Journal of Colloid and Interface Science 188 (1997) 68-74. 13. V. Noethig-Laslo and Lj. Brečević: Mode and sites of incorporation of divalent cations in vaterite. Journal of Chemical Society, Faraday Transaction 94 (1998) 2005-2009. 14. V. Nöthig-Laslo and Lj. Brečević: An EPR study of Cd2+ incorporation in vaterite. Physical Chemistry and Chemical Physics 1 (1999) 3697-3700. 15. V. Nöthig-Laslo and Lj. Brečević: An EPR study of primary paramagnetic centres in Cd2+-doped vaterite. Physical Chemistry and Chemical Physics 2 (2000) 5328-5332. 16. D. Kralj, Lj. Brečević and A.E. Nielsen: Crystal Growth of Vaterite. The Effect of Temperature and Ionic Strength. In: Industrial Crystallization '90, A. Mersman ed. (Garmish-Partenkirchen, F.R. Germany, 1990) p. 267-272. 17. D. Kralj, Lj. Brečević and A.E. Nielsen: Dissolution Kinetics of Vaterite. In: Industrial Crystallization '93, Z.H.Rojkowski ed. (Warsaw, Poland, 1993) p. 4-119 - 4-124. 18. N. Vdović and D. Kralj: The Influence of Some Naturally Occurring Minerals on the Precipitation of Calcium Carbonate Polymorphs. In: 35th CIESM Congress Proceedings (Dubrovnik, Croatia, 1998) Volume 35(1), p. 300-301. (Rapp. Comm. int. Mer Médit., 35, 300-301) 19. N. Vdović and D. Kralj: CaCO3 precipitation from artificial sea water - The influence of organic and inorganic additives. In: Rapports de la commission internationale pour l'exploration scientifique de la mer Méditeranée 36. , Monaco 2001, p. 171. 20. Lj. Brečević and A.E. Nielsen: Solubility of Amorphous Calcium Carbonate. Journal of Crystal Growth 98 (1989) 504-510. 21. Lj. Brečević and A.E. Nielsen: Precipitation and Properties of an Amorphous Calcium Carbonate. In: Industrial Crystallization '90, A. Mersmann, ed. (Garmisch-Partenkirchen, Germany, 1990) p. 241-246. Laboratory for Precipitation Processes Division of Material Chemistry List of available equipment Electronic Particle Analyzer, Coulter Counter, Mo. TA Electronic Particle Analyzer, Coulter Counter, Multisizer II Laser Particle Analyzer, Brookhaven Mo. 4700 C Equipment for TIRF spectroscopy (Total Internal Reflectance Fluorescence) Thermobalance TG 50 with TC 11 Processor, Mettler Specific Surface Area Analyzer, Micromeritics, Gemini FT-IR Spectrometer, ATI Mattson, Genesis Series UV-VIS Spectrophotometer, Beckman Mo. 34 Optical Microscope, Ernst Leitz, Wetzlar, Orthoplan Inverted Optical Microscope, Ernst Leitz, Wetzlar Analytical Balance, Sartorius Analytical Balance, Chyo Balance Corp., JL-200 Analytical Balance, Chyo Balance Corp., JL-3000 Conductivity Meter, Radiometer CDM 3 pH-meter, Radiometer, PHM 26 pH-meter, Radiometer, PHM 64 pH-meter, Radiometer, ION 85 Potentiometric Titrator, Radiometer, Titrigraph pH-meter/pH(Chemi)-stat Controller, Radiometer, PHM 290 Autoburettes ABU 901 Micro centrifuge, Hawskley Mo. E Water bath shaker, Brunswick Refrigerated bath circulator, Haake Ultrasonic bath, Iskra Ultrasonic homogenizer, Branson Sonifier II Stirrers with speed/torque controller, Heller, GKH List of equipment avilable in other laboratories in the Rudjer Boskovic Institute Atomic Absorbtion Spectrophotometer, Perkin-Elmer X-ray Diffractometer, Philips ESR Spectrometer, Varian E9 -source 60Co Transmission Electron Microscope, Zeiss EM 10A