The Rate Determining Step
advertisement
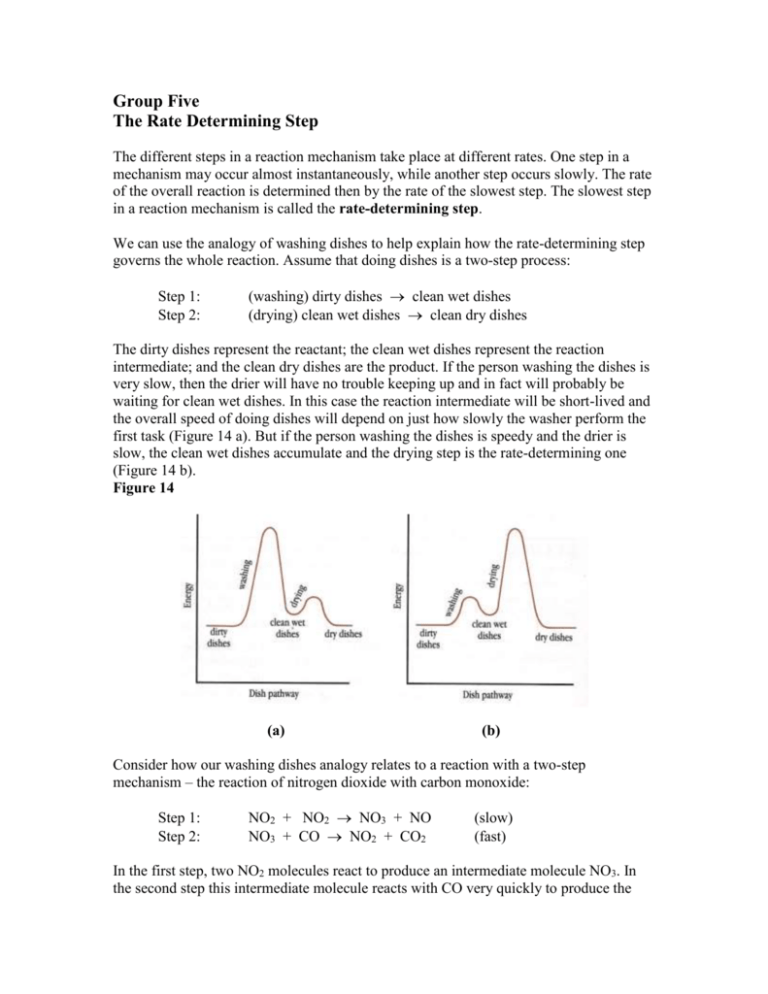
Group Five The Rate Determining Step The different steps in a reaction mechanism take place at different rates. One step in a mechanism may occur almost instantaneously, while another step occurs slowly. The rate of the overall reaction is determined then by the rate of the slowest step. The slowest step in a reaction mechanism is called the rate-determining step. We can use the analogy of washing dishes to help explain how the rate-determining step governs the whole reaction. Assume that doing dishes is a two-step process: Step 1: Step 2: (washing) dirty dishes clean wet dishes (drying) clean wet dishes clean dry dishes The dirty dishes represent the reactant; the clean wet dishes represent the reaction intermediate; and the clean dry dishes are the product. If the person washing the dishes is very slow, then the drier will have no trouble keeping up and in fact will probably be waiting for clean wet dishes. In this case the reaction intermediate will be short-lived and the overall speed of doing dishes will depend on just how slowly the washer perform the first task (Figure 14 a). But if the person washing the dishes is speedy and the drier is slow, the clean wet dishes accumulate and the drying step is the rate-determining one (Figure 14 b). Figure 14 (a) (b) Consider how our washing dishes analogy relates to a reaction with a two-step mechanism – the reaction of nitrogen dioxide with carbon monoxide: Step 1: Step 2: NO2 + NO2 NO3 + NO NO3 + CO NO2 + CO2 (slow) (fast) In the first step, two NO2 molecules react to produce an intermediate molecule NO3. In the second step this intermediate molecule reacts with CO very quickly to produce the products NO2 and CO2. Increasing the concentration of CO will make Step 2 take place faster, but will have little effect on the speed of the overall reaction because Step 1 is the rate-determining step! However, increasing the concentration of NO2 will speed up the overall reaction because it is a reactant in Step 1, the rate-determining step. Knowing the mechanism of a reaction provides a basis for predicting the effect of the concentration of a reactant on the overall rate of a reaction. If the reaction mechanism is unknown, then experiments must be done to determine the effect. Demo: Materials: retort stand, 3 funnels with different bore diameters, rings to hold funnels, beaker, graduated cylinder, coloured water to make demonstration more visible, stopwatch Demonstration: Measure the time required for x mL of water to pass through each of the three funnels. These values can then be converted to rates (ml/s). Next, arrange the 3 funnels in consecutively (in any order – to really convince students let them try all possible orders to see that the result remains the same) so that water flowing out of one will flow into the next. Then ask students to predict (and explain their predictions) what they think the rate will be for water to flow through all three funnels. Measure the time it takes for the water to flow through all of the funnels (start timing when water begins flowing out of last funnel and into beaker) and convert this to a rate. Compare the rate of water flow through each individual funnel to the rate of water flow through the series of funnels. Sample Questions 1. Which rate in the steps of a reaction mechanism determines the rate of a reaction? 2. An important function for managers is to determine the rate-determining steps in their business processes. In a certain fast-food restaurant is takes 3 minutes to cook the food, 1.5 minutes to wrap the food, and 5 minutes to take the order and make change. How would a good manager assign the work to four employees? 3. The reaction 2A + B + C D takes place through the following mechanism: Step 1: Step 2: Step 3: A + B AB AB + A A2B A2B + C D (fast) (slow) (fast) Predict and explain what affect on the rate of the overall reaction an increase in the concentration of each of the following would have: (a) substance A (b) substance B (c) substance C 4. Consider the following reaction mechanism: Step 1: Step 2: Step 3: Step 4: A2 2A 2A + 2B 2AB 2AB + C2 2ABC 2ABC 2AC + 2B (fast) (slow) (fast) (fast) (a) Write the net equation for the overall reaction. (b) Would the speed of the overall reaction increase if additional amounts of A, B, or C were added to the container? Explain why or why not. 5. The following potential energy diagram describes the reaction between hydrogen bromide and oxygen gas: 4 HBr (g) + O2 (g) 2 H2O (g) + 2 Br2 (g) (a) What is the H for the entire reaction? (b) Which step is the ratedetermining step for this three step reaction? (c) What is the Ea for the ratedetermining step? (d) What is the Ea for the reverse direction of the rate-determining step? (e) What is the H for the entire reaction in the reverse direction?









