Conservation of Cookie Matter
advertisement

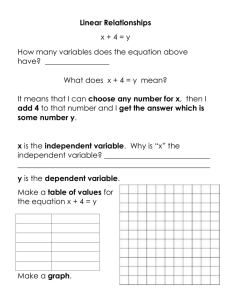
Prior Knowledge: Guiding Questions: Learning Objectives: Benchmarks: SC.4.P.8.3 Conservation of Cookie Matter PLUS SC.4.P.8.3: Explore the Law of Conservation of Mass by demonstrating that the mass of a whole object is always the same as the sum of the masses of its parts. SC.4.N.1.4: Attempt reasonable answers to scientific questions and cite evidence in support. SC.4.N.1.5: Compare the methods and results of investigations done by other classmates. SC.4.N.1.6: Keep records that describe observations made, carefully distinguishing actual observations from ideas and inferences about the observations. The students will be able to: • Describe a closed system; • Explain why mass is not changed in a closed system, regardless of what is done to the object; • Make observations and inferences based on those observations; • Distinguish between observations and inferences; • Compare methodology of different groups and explain differences in results across the groups. Guiding questions are broad questions that the teacher can use to “guide” the student learning process and can be revisited at any point in the lesson. Teacher Note: In this activity, students will be learning a very important foundational Science concept: the mass of a closed system will not change, no matter what you do to the parts, as long as the system remains closed. This science law is important to understanding in every field of science. The questions below are important questions that the teacher needs to keep in mind as the investigation unfolds. • Is it possible for something to change and yet remain the same? (Yes, no matter what you do to an object in a closed system, the mass will always stay the same.) • How can human actions impact the results of a scientific investigation? (If the scientist doesn’t carefully control the experiment, different results can result.) • How do changes affect the mass of an object? Students should know that inferences are based on observation They should also know how to compare the observations made by different groups using the same tools and seek reasons to explain the differences across groups. They should also know how to measure and compare the mass of objects. (All are grade 3 benchmarks). Students should also know the states of matter (liquid and solid) for Part 3. Submitted by: Wendy Shelden, TASK-Force Resource Guide, Brevard Public Schools Page 1 of 9 SC.4.P.8.3 Conservation of Cookie Matter PLUS Materials: Classroom Management: Each of the parts of this activity could be used as your whole-class Science Project and individual/team projects. Part 1 should take 1-2 class periods (depending on whether you are introducing the Science Fair process). Part 2 (dried foods) should take 1 class period. Part 3 (ice pops) should only take one class period, but needs to be separated by several hours to allow time for freezing. Part 4 (grow gator) only takes about 15 minutes to set it up, then 3-5 minutes a day to check for the next week. One class period at the end would be ideal to discuss all the investigations. The most important part of each part of this investigation is controlling the variables—this is what causes most of the differences in data. Some areas to watch out for: Cookies/Dried Food Items (parts 1-2): The cookies must stay in the sealed plastic bags—even while the students are breaking them and crushing them. That way you are measuring the combined mass of the pieces and none escaped. If you open and close the bag, you are letting air in and out, and we know that air has at least a little bit of mass. To model how important this step is, do the experiment once when you take the cookies out to break them apart. When you weigh them, the mass is different because part of the cookie mass was left on the table you smashed them on. This shows the students the importance of controlling your variables. If the bag gets a hole, that’s an invalid trial. Ice Pops (part 3): You have to wipe off the condensation that is frozen to the outside of the pop, and it will try to re-condense as soon as you wipe it off. So you have to be very quick about getting the condensation off before finding the mass. Gator in the bottle (part 4): Make sure you place the bottle in the same spot on the scale each time. Part 1: ~Cookies (2 per student) ~Zippered plastic bags ~Digital scale Part 2: ~Zippered plastic bags ~Digital scale Part 3: ~Ice Pops (1 per student) ~Masking tape ~Permanent marker ~Digital scale ~Freezer Part 4: ~1 empty 2-liter bottle with lid ~water to fill bottle ~1 “grow gator” ~Digital scale Engage: Make up zippered plastic bags with 2 whole cookies per bag. Only make enough bags of whole cookies for each student in the class. Also make a few bags with 2 cookies broken into pieces and a few with 2 cookies completely pulverized in to crumbs. As the students come into class, tell them to grab a bag of cookies. They will probably all grab the whole cookies. Go over to get your bag of cookies and complain loudly that there are only little pieces left. Try to exchange bags with the students. If they won’t exchange with you ask, “Why not?” Try to get the students to say there are more cookies in the whole bags than the pieces bags. If no one says that, then say, “It’s not fair, your bags have more.” When the kids either laugh or tell you they are all the same mass, challenge them to help you design a Science investigation to check their hypotheses. Submitted by: Wendy Shelden, TASK-Force Resource Guide, Brevard Public Schools Page 2 of 9 SC.4.P.8.3 Conservation of Cookie Matter PLUS These directions are for Part 1: Cookies. Part 2, 3, and 4 use this same format, but you just need to make the appropriate changes based on the new objects being investigated. Teacher Note: There is a Student Inquiry Lab Report (included at the end of the lesson). It is intended as a template to help the students keep track of the parts of a science experiment. Explore: 1. Explain that you are going to design an experiment to test whether the mass of an object changes when you break it apart. The first thing to do is identify the variables. The independent variable is the thing you are changing in the experiment. What are we changing? (the number of pieces: 1, 5, or many) The dependent variable is what we are measuring. What are we measuring? (the mass of the cookies) Control variables are all the other things in the experiment that must be kept exactly the same so that we have results we can depend on (scientifically valid results). Ask the students to list the things that have to be kept the same for a fair (valid) test. Your final variables should look something like this: Variables: Independent-Number of pieces of the cookies (crushed, pieces, or whole) Dependent-Mass of the cookies Controlled-number of cookies you started with; same bag; same amount of air in bag; same scale each time; same person reading the scale each time; repeated trials (everyone in the class will do it), same cookies for each round of trials, same kind of cookies, etc. 2. The second step is using the variables to write a question. Model how to write the following question: Question: How does the number of pieces (independent variable) affect the mass (dependent variable) of the cookies (topic of experiment)? 3. Model writing an “If…, then…” hypothesis. Everyone does NOT have to have the same hypothesis, but they do all have to have a justification for their hypotheses. Hypothesis: If I measure the mass (dependent variable) of cookies that are whole or in pieces (control variable), then the whole cookie (independent variable condition that the student thinks will have the most change) will have a mass that is 5 grams more (needs quantity) than the cookie pieces. I think this will happen because the cookie loses mass as it is broken. (Justification) 4. Now you are ready to design the Procedure. Work the students through the steps. Guide them to a fair test by using the control variables. Your Procedure might look something like this: Procedure: 1) Place 2 whole cookies in a zippered bag. Seal the bag after getting most of the air out. 2) Find the mass of 2 whole cookies in a sealed bag. Record on data chart. 3) Without opening the bag, break the cookies into bite-sized pieces. 4) Using the same method and scale as Step 2, find the mass of the broken pieces in the sealed bag. Record. 5) Without opening the bag, pulverize the cookies. 6) Using the same method and scale as Step 2, find the mass of the pulverized cookies in the sealed bag. Record. Submitted by: Wendy Shelden, TASK-Force Resource Guide, Brevard Public Schools Page 3 of 9 Explore, Part 2: Explore, (continued) SC.4.P.8.3 Conservation of Cookie Matter PLUS 5. The next step after designing a Procedure is making a Data Chart. Again, guide the students through constructing their own Data Chart. Use the one on the Student Inquiry Lab Report as a guide for you. 6. Once you’ve designed the Data Chart, have the students begin their trials. Monitor the scales to make sure they are following the procedure. Do not let anyone throw out the bag of cookies at the end. 7. Check the results with the students. Everyone should have results showing that the mass didn’t change at all. If some have differences, ask another student to inspect that student’s bag, looking for holes. Then have the first student go through the procedure again with a new bag of cookies, with the second student acting as Observer to verify that the control variables were controlled. This usually helps point out any errors. 8. Once all the results are verified, guide the students to an understanding that the zippered bag creates a “closed system.” As long as the bag isn’t opened, the mass of the bag and its contents will not change, regardless of what you do to it. As soon as you open the bag, you’ve opened the system, and the mass could change. 9. Draw conclusions about how the size of the pieces affected the mass of the cookies. Conclusion: My data (does or does not) support my hypothesis. My hypothesis stated that ____(give full hypothesis)_____ , and my data showed that the mass of the cookies didn’t change at all. I think this happened because the zippered bag created a closed system. As long as it was closed, nothing could change the mass of the cookies. 10. If you are using this as a Science Fair process, continue on with the Summary. Then proceed to Part 2. Part 2: Dry Food Give each child a new zippered bag. Tell them to take it home and put a dry food item in the bag. Some suggestions are chips and cereal. Explain that they will be repeating the investigation with items of their choice tomorrow. On the next day, go over the variables, question, hypothesis, and procedure from Part 1. Ask, “What do we need to change? The only change should be to substitute the new item for the word cookie. HINT: In the Science notebook, you can have the students write the new question and hypothesis, but for the procedure, just write: “Follow procedure from Part 1.” Also, remind the students to think about their hypothesis. Caution: Do not tell them that the mass should be the same—let them come to that understanding on their own. Don’t worry if some continue to think that the mass will change. The fact that the mass stays the same is counter-intuitive. The students will need lots of evidence to get them to change their minds, which they will get with Parts 2, 3, and 4. Follow Steps 7-8 above to test and discuss the results. Again, if anyone has different results, assign an Observer. Submitted by: Wendy Shelden, TASK-Force Resource Guide, Brevard Public Schools Page 4 of 9 Explore, Part 4: Explore, Part 3: SC.4.P.8.3 Conservation of Cookie Matter PLUS Part 3: Ice Pops Give each student a room temperature Ice Pop. Show them how to fold the ice pop in half, wrap masking tape around the ice pop several times to hold it in half, then use a permanent marker to write his/her name on the pop. Explain that you are going to be freezing the ice pop. This time, the independent variable is the state of matter (liquid or solid). Guide them in changing the question and writing a new hypothesis. Again, don’t tell them what to expect. Students know that ice expands, so they will expect the mass to increase. Ask if the procedure needs to change. (Yes, we aren’t breaking it into pieces; we are putting it in a freezer for ___ hours. Also, they will need to add a step for wiping off all the condensation/frost on the outside of the frozen ice pop before weighing it.) Test the ice pops and discuss the results as you did for Parts 1 & 2. Part 4: Grow Gator This investigation is done by the teacher only. Fill a 2-liter bottle with water. Push one “grow gator” (available at www.stevespanglerscience.com) into the bottle and replace the lid tightly. Make sure you dry off any external water drops! Place the bottle on the scale, placing it directly in the middle of the scale. Record the mass of the bottle on the board, then place the bottle up high on a shelf where everyone can see it, but no students can touch it or mess with it. Explain that we will be leaving the bottle on the shelf, but taking it down each morning to find the mass and make any observations. Explain to the students that the gator is a “grow gator.” Don’t tell them anything else! Guide them in changing the question and writing a new hypothesis. Again, don’t tell them what to expect. This is where they really have to face their misconceptions. Each morning, take down the bottle, weigh it in exactly the same place and record the results on the board (and in the Science notebooks). Encourage the students to also record any observations they make. (Possible observations: the gator is getting larger; the water level is dropping.) At the end of the week, find the mass of the bottle one more time. The mass should not have changed, even though the gator got noticeably larger. Submitted by: Wendy Shelden, TASK-Force Resource Guide, Brevard Public Schools Page 5 of 9 Evaluate: Elaborate: Explain: SC.4.P.8.3 Conservation of Cookie Matter PLUS As you discuss the results of all the investigations, ask the students the following questions: Why did the mass stay the same, even though the gator got noticeably larger? (The 2L bottle is a CLOSED SYSTEM. As long as it remains closed, nothing can get in or out—the mass (amount of matter) inside remains the same, regardless of what physical changes take place within the bottle. The gator absorbed lots of water that made its size bigger, but there was still the same amount of mass in the bottle.) Why did the mass stay the same, even though the water level dropped? (same as above) Compare the “grow gator” experiment to the ice pops and cookie experiment. Why did you get the same results? (All are examples of CLOSED SYSTEMS—nothing gets in and nothing gets out, so the mass remains the same.) Why did we occasionally get different results? (Sometimes it was that we couldn’t control every variable—especially with the ice pops condensation problem. Most of the time, though, it was due to human error.) Go over the observations they made during Part 4. Lead a discussion on whether they are all observations, or if some inferences crept in. If they wrote, “The gator is getting larger.”—that’s an observation. If they wrote, “The mass is increasing.” or “I think…..”—those are inferences. If you used this as a Science Fair project introduction, add these questions: Is there a component of the Scientific Method that we didn’t do? (Yes, Research) What could we have researched to help us make a better Hypothesis? (Accept all plausible answers; some possibilities: mass, scales, how cookies are packaged to prevent breakage.) Ask the students to design their own investigation testing the mass in a closed system. Some possibilities: cereal and milk an apple as it decays (sealed in a plastic bag) Diagnostic and/or formative assessments are embedded within the lesson for the purpose of identifying preconceptions and driving instruction so the students have increased opportunities to learn the important ideas related to the topic. Summative Assessment: Give students the “Seedlings in a Jar” probe from Page Keeley’s Uncovering Student Ideas in Science, Volume 1. This is another example of a closed system. Submitted by: Wendy Shelden, TASK-Force Resource Guide, Brevard Public Schools Page 6 of 9 SC.4.P.8.3 Conservation of Cookie Matter PLUS Student Inquiry Lab Report Use this format to plan out your investigation. Record all parts in your Science notebook or Science Fair log. Identify variables: Independent variable (condition you are changing)_______________________ Dependent variable (what are your measuring)____________________________ Control variable (all other conditions to be kept the same)___________________ ______________________________________________________________________ Problem: How does (independent variable) affect the (dependent variable) of (controlled variable)? How does __________________________ affect the ______________________ of __________________________________? Hypothesis: Use your variables again: If I measure the (dependent variable) of (control variable), then the (independent variable) will (do what?). If I measure the __________________________________ of ___________________, then the ___________________________________ will ________________________ _________________________________________________________. I think this will happen because ________________________________________________________. Procedure/Materials: Write on another piece of paper. Data/Results: What is the best way to record the data you observed? Chart, graph, illustrations? Use a separate sheet of paper for this so you have plenty of room. Conclusions: My data (does or does not) support my hypothesis. My hypothesis stated that ____(give full hypothesis)_____ , and my data showed that the mass of the cookies didn’t change at all. I think this happened because ______________________ ______________________________________________________________________. Share and Communicate: What is the best way to Communicate your experiment and Results with the class or others? Submitted by: Wendy Shelden, TASK-Force Resource Guide, Brevard Public Schools Page 7 of 9 SC.4.P.8.3 Conservation of Cookie Matter PLUS Conservation of Cookie Matter Data Chart Mass of Whole cookies (in grams) Mass of Large Chunks (in grams) Mass of Crumbs (in grams) Trial 1 Trial 2 Trial 3 Trial 4 Trial 5 Trial 6 Trial 7 Trial 8 Trial 9 Trial 10 Submitted by: Wendy Shelden, TASK-Force Resource Guide, Brevard Public Schools Page 8 of 9 SC.4.P.8.3 Conservation of Cookie Matter PLUS Conservation of Ice Pop Matter Data Chart Mass of room temp Pop (in grams) Mass of frozen Pop (in grams) Trial 1 Trial 2 Trial 3 Trial 4 Trial 5 Trial 6 Trial 7 Trial 8 Trial 9 Trial 10 Submitted by: Wendy Shelden, TASK-Force Resource Guide, Brevard Public Schools Page 9 of 9