Dated
advertisement
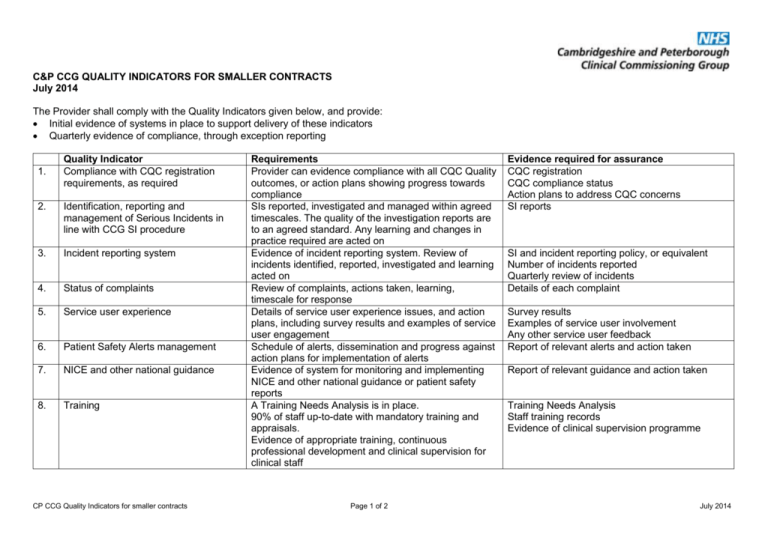
C&P CCG QUALITY INDICATORS FOR SMALLER CONTRACTS July 2014 The Provider shall comply with the Quality Indicators given below, and provide: Initial evidence of systems in place to support delivery of these indicators Quarterly evidence of compliance, through exception reporting 1. Quality Indicator Compliance with CQC registration requirements, as required 2. Identification, reporting and management of Serious Incidents in line with CCG SI procedure 3. Incident reporting system 4. Status of complaints 5. Service user experience 6. Patient Safety Alerts management 7. NICE and other national guidance 8. Training CP CCG Quality Indicators for smaller contracts Requirements Provider can evidence compliance with all CQC Quality outcomes, or action plans showing progress towards compliance SIs reported, investigated and managed within agreed timescales. The quality of the investigation reports are to an agreed standard. Any learning and changes in practice required are acted on Evidence of incident reporting system. Review of incidents identified, reported, investigated and learning acted on Review of complaints, actions taken, learning, timescale for response Details of service user experience issues, and action plans, including survey results and examples of service user engagement Schedule of alerts, dissemination and progress against action plans for implementation of alerts Evidence of system for monitoring and implementing NICE and other national guidance or patient safety reports A Training Needs Analysis is in place. 90% of staff up-to-date with mandatory training and appraisals. Evidence of appropriate training, continuous professional development and clinical supervision for clinical staff Page 1 of 2 Evidence required for assurance CQC registration CQC compliance status Action plans to address CQC concerns SI reports SI and incident reporting policy, or equivalent Number of incidents reported Quarterly review of incidents Details of each complaint Survey results Examples of service user involvement Any other service user feedback Report of relevant alerts and action taken Report of relevant guidance and action taken Training Needs Analysis Staff training records Evidence of clinical supervision programme July 2014 9. Clinical Audit programme 10. Workforce and staffing 11. Safeguarding 12. DNAR forms 13. Health Care Acquired Infections 14. Pressure ulcers 15. Admission, Discharge and Transfer of care 16. Care of Equipment Audits undertaken to evidence CQC compliance, and in Audit reports showing rationale for audit, learning response to incident / complaints intelligence and action plans Evidence of re-audits Establishment review undertaken 6-monthly or at any Establishment review major service development. Workforce metrics such as sickness, turnover, Staffing review at start of all shifts against agreed vacancy rates, use of temporary staff. establishment (dependent on patient numbers and Escalation policy acuity) to identify any gaps and allow escalation of Recruitment policy concerns. Evidence of staff records showing qualifications All staff appropriately recruited, trained and qualified for the role undertaken. Assurance of robust systems for safeguarding children Safeguarding policy and vulnerable adults, including analysis and delivery Details of any reported safeguarding issues of safeguarding training, and compliance with the principles of the “Delivering Dignity” report Compliance of DNAR forms and care pathways are in Number of DNAR forms in place. place. Annual DNAR audit Assurance of robust systems for HCAI which ensure Policy for managing HCAI patient safety, including relevant training. Reporting 90% of staff trained in relevant HCAI procedures, and Root cause analysis investigation of any MRSA including hand washing bacteraemia and C Difficile infection. Reporting and Report on management of, and learning from, management of outbreaks and infections of any MRSA bacteraemia and C Difficile infection significance Report on management of, and learning from, any outbreaks and infections of significance Number of grade 2, 3 and 4 PUs, with details of root Pressure ulcer guidance cause analysis used to generate learning, action plans Numbers of PUs by grade for improvement and evidence of implementation of changes required. Assurance of robust policies, procedure and criteria for Admission, Discharge and Transfer of care Admission, Discharge and Transfer of care protocol showing a collaborative approach with other stakeholders Evidence of an equipment protocol and regular checks Schedule for equipment checks of equipment. Audit of equipment checks Evidence should be submitted to the C&P CCG Contract lead, XXXXXX CP CCG Quality Indicators for smaller contracts Page 2 of 2 July 2014