administered substances - Harvard Medical School
advertisement

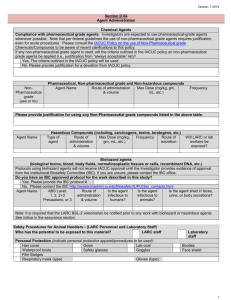
Harvard Medical Area Page 1 of 5 Protocol # Revised 5/27/14 Harvard Medical Area (HMA) Standing Committee on Animals – “Established in 1907” 180 Longwood Avenue, Suite 113 Boston, MA 02115 Tel: 617-432-3192 Fax: 617-432-3169 http://www.hms.harvard.edu/orsp/ iacuc@hms.harvard.edu I. ADMINISTERED SUBSTANCES 1. Will you administer any substances to your animals during the course of the life of the animal(s)? Yes____No____ If "NO", proceed to Section J 2. If “YES”, list all administered substances here and categorize (refer to http://toxnet.nlm.nih.gov/): Do NOT include analgesia/anesthesia here, include as appropriate in Section B or Section J Specify each substance: a. Chemical carcinogen: b. DEA Controlled substance: c. Toxic substance: d. Radioisotope: license #: e. Microbiological agent*: f. Human or animal cell lines**: g. Human embryonic stem cells (hESC)*** h. Other (any other agent, EXCEPT anesthesia or analgesia): HCCM must be contacted if you will be using substances potentially harmful to humans in the animal facilities, see the Mandatory Risk Disclosure page. 3. For substances potentially harmful to either animal subjects or staff: a. The use of *microbiological agents must be approved by the Committee on Microbiological Safety (COMS) or Partners Institutional Biosafety Committee (PIBC). Contact a Biosafety Officer at EH&S at 617-432-1720 (HMS) or 800-825-5343 (BWH) for the necessary forms. BWH investigators must obtain approval from the Partners IBC; registrations can be completed online: https://insight.partners.org or contact the PIBC for assistance at pibc@partners.org Provide the COMS/PIBC Registration # b. The use of **human or NHP tissues or cells must be approved by COMS or PIBC. Contact a Biosafety Officer at EH&S at 617-432-1720 (HMS) or 800-825-5343 (BWH) for the necessary forms. BWH/MGH investigators must obtain approval from the Partners IBC; registrations can be Page 1 of 5 Harvard Medical Area Page 2 of 5 Protocol # Revised 5/27/14 completed online: https://insight.partners.org or contact the PIBC for assistance at pibc@partners.org Provide the COMS/PIBC Registration # c. Protocols involving the use of ***hESC must also be sent to the Harvard University Embryonic Stem Cell Research (ESCRO) Committee for review and approval (617-495-9829) and the Office of Research Compliance must be notified (617-432-3884). BWH PI’s – Contact the Partner’s ESCRO Committee (617-424-4171). Children’s PI’s – Contact the Children’s ESCRO Committee d. IACUC approval CANNOT be granted until ESCRO approval is in place. Have you submitted an ESCRO protocol application? Yes____No____ List your ESCRO Protocol Number here: ___________________ 4. Specify substances potentially contaminated with infectious agents (BOTH human- and animal-derived tumor or tissue cell lines must be tested for biological contaminants prior to use. For further information and details on how to proceed, see https://ARCM.med.harvard.edu/secure/1_getting_started/gs_cell_lines.htm: a. Animal Cell Lines: b. Tumor Cell Lines: c. Biologics (Monoclonal Antibodies, serum-derived products): 5. Specify the following for each substance listed above (copy this table and complete separately for each substance): AGENT: VERTEBRATE SPECIES to which agent will be administered: a. Volume, Concentration, Route of administration: b. Anatomic Site of injection(s) including tissue layer (IM, SQ, ID, IP, IC, etc.) (if applicable): c. Number of Injection(s) (if applicable): d. Effects of the agent or substance on research animal: e. Length of survival time after initial administration: f. Will the animal experience pain, distress or illness as a result of the substance administered? Yes No Page 2 of 5 Harvard Medical Area Page 3 of 5 Protocol # Revised 5/27/14 If "YES", describe the procedures to alleviate the pain, distress or illness: g. Is this compound pharmaceutical grade (USP)? Yes No If “NO” briefly describe the following, as related to the preparation(s) you will be administering: Chemical purity (e.g. HPLC grade, life-science grade): Methods to ensure sterility (e.g. micron filtration, sterile diluents, sterile containers): pH range (as appropriate for the material being delivered): Storage (e.g. container type, temperature): Labeling (e.g. compound name, concentration, preparation date): Expiration date: 6. Specify the following for all HAZARDOUS/INFECTIOUS agents: You must complete the following section for all studies involving HAZARDOUS/INFECTIOUS agents: Agent Used Chemical Carcinogen: Agent Used Toxic Substance: Existing COMS Biosafety Agent Used Microbiological Agent: or PIBC #: level: License #: Half-life: Agent Used Radioisotope: Route of Administration: Blood Saliva Urine Feces Check Shed in: Location where agent Duration of Animal will be administered: Survival Post treatment: Building: Room: Special Housing Request: Animal Housing Location Post-treatment: Has contact with ARCM and EH&S been established for use of hazardous agents? Yes No State the terms of, or describe the agreement with HCCM and EH&S on procedures Page 3 of 5 Harvard Medical Area Page 4 of 5 Protocol # Revised 5/27/14 and precautions for use of hazardous agents: 7. Pharmaceutical-grade compounds a. Will you be using compounds that are not pharmaceutical-grade? Yes____No____ In keeping with the USDA and OLAW guidance, it is the policy of the IACUC that pharmaceutical-grade compounds, including diluents and carriers, must be used in experiments instead of non-USP drugs or reagents, even in acute procedures. Non-pharmaceutical-grade chemical compounds, diluents and carriers may only be used after specific review and approval by the IACUC. The IACUC may consider scientific necessity or non-availability of an acceptable veterinary or human pharmaceutical-grade product as sufficient reasons for accepting the use of non-pharmaceutical grade drugs or diluents in animal experiments. Cost savings alone is not a sufficiently compelling reason to justify use of a potentially ineffective or toxic nonpharmaceutical-grade compound. b. If you are administering ANY non-pharmaceutical grade drugs, diluents or carriers, list each in the table below and check appropriate box a., b., or c. Compound X Identify need for non-USP compound a. Non-commercially available experimental compound b. Only non-USP grade is commercially available and no other alternatives exist c. USP grade drug or alternative is available, but a non-USP grade is requested c. If pharmaceutical-grade compounds are available and you are NOT administering pharmaceuticalgrade compound(s) to your animal(s), (c is checked) you must also complete the detailed scientific justification column below. The IACUC expects researchers to justify the use of a non-pharmaceutical grade drug, compound, diluent, or carrier and to demonstrate consideration (and rejection) of pharmaceutical grade alternatives for experimental reasons. Reasons related to the cost of USP grade products are generally deemed insufficient on those grounds alone to justify substitution of non-pharmaceutical grade alternatives for USP drugs and reagents. Provide sufficient detail and present the respective advantages and disadvantages of the pharmaceutical and non-pharmaceutical-grade compound/chemicals. • Describe how rejected alternatives will negatively impact outcomes or measurements. • Describe how experimental logistics have influenced the choice of the chosen agent. Page 4 of 5 Harvard Medical Area Page 5 of 5 Protocol # Revised 5/27/14 • Address any issues of animal or human safety, compound efficacy related to the experimental aim, and address the possibility of inadvertent introduction of research-complicating variables. Provides detailed scientific justification Page 5 of 5