Hazardous / Non-Pharmaceutical / Controlled Substances

APPENDIX C:
Hazardous Agents/Non-Pharmaceutical
Grade Compounds/Controlled Substances
I.
Hazards
1) Biological Agents ☐
Yes
☐
No
☐
Human Cell Lines, Tumors, Blood
☐
Infectious Agents
☐
rDNA
☐
Other: Click here to enter text.
a) List agents, source and indicate biosafety level for each. Add rows if needed
Agent Source Biosafety Level b) Complete table for each agent separately. add rows if needed
Agent Route & Site Dose Frequency c) Has an application been submitted to the IBC or relevant committee for the use of agent?
☐
Yes – provide date of approval and reference number: Click here to enter text.
☐
No – explain status of application: Click here to enter text.
d) If derived from rodents or humans, has the material been tested for pathogens as described in IACUC
Policy 017 Transplantable Cell Lines ? Discuss testing options with the Attending Veterinarian. Results must be approved by the Attending Veterinarian prior to introduction to animals.
☐
Yes – Please attach certification of testing
☐
No – Provide justification: Click here to enter text.
e) Describe what procedures will be taken to minimize exposure risk. Include PPE, equipment use, frequency of use, and any other relevant information.
2) Radioactive Material / Lasers a) Type
☐
Yes
☐
No
☐
Radioisotopes
☐
Lasers
☐
X-ray
☐
Irradiator
☐
Other: Click here to enter text.
b) List isotopes c) Indicate where work will be conducted: f) Describe what procedures will be taken to minimize the exposure risk. Include PPE, equipment use, frequency of use, and any other relevant information.
Page 1 of 3
Revised 2015
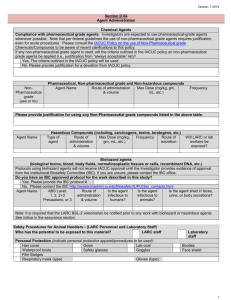
3) Chemical Agents / Drugs / Controlled Substances
Refer to IACUC Policy 015 - Use of Non-Pharmaceutical Grade Compounds and/or
Expired Medical Materials a) List agents, source and indicate biosafety level for each. Add rows if needed
Agent Dose & Route Frequency
☐
Yes
☐
No
Pharmaceutical Grade
(Yes, No or N/A) b) Where will materials be stored? c) If compounds are non-pharmaceutical provide a justification why compounds will be used? d) Source: e) Describe the preparation, approximate pH, storage and stability, shelf life, sterility & pyrogenicity of each compound. g) Describe what procedures will be taken to minimize the exposure risk. Include PPE, equipment use, frequency of use, and any other relevant information.
4) Other Hazards a) Substance and Hazard:
☐
Yes
☐
NO b) Locations substance will be stored: c) Describe what procedures will be taken to minimize the exposure risk. Include PPE, equipment use, frequency of use, and any other relevant information.
II.
Adverse effects
1) Explain how animals will be monitored to detect adverse effects (if any) such as reactions, infections, behavioral changes, etc.
2) Identify the proposed actions to be taken for each adverse effect
Page 2 of 3
Revised 2015
Revised 2015
Page 3 of 3