LabE_AtomicSpectra_o..
advertisement
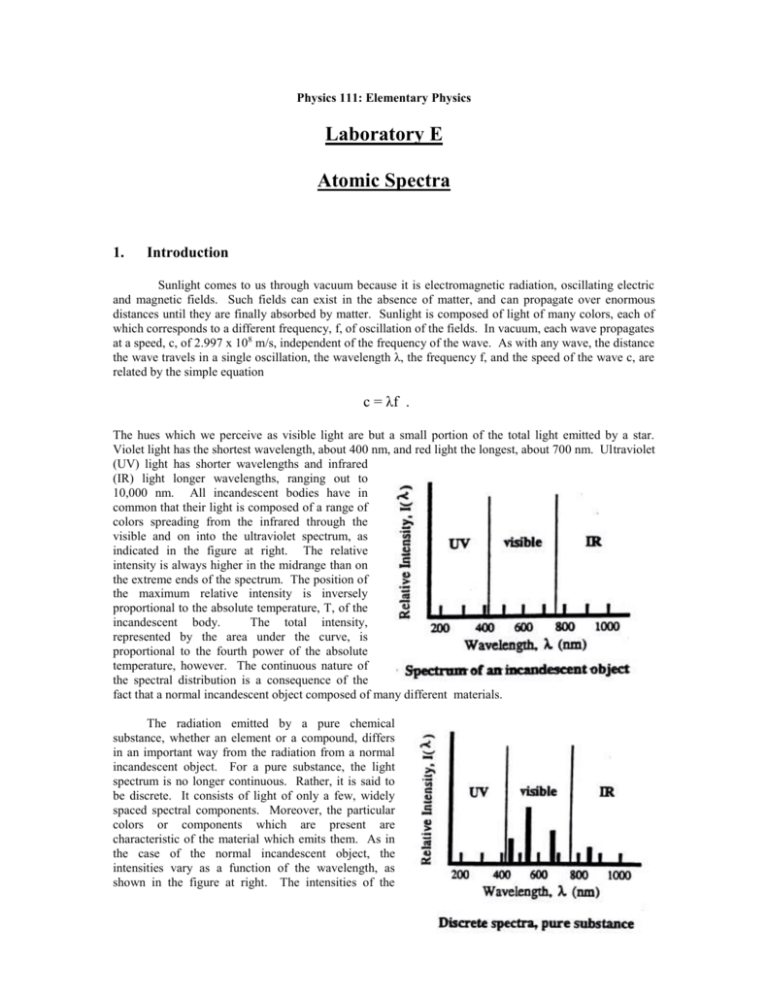
Physics 111: Elementary Physics Laboratory E Atomic Spectra 1. Introduction Sunlight comes to us through vacuum because it is electromagnetic radiation, oscillating electric and magnetic fields. Such fields can exist in the absence of matter, and can propagate over enormous distances until they are finally absorbed by matter. Sunlight is composed of light of many colors, each of which corresponds to a different frequency, f, of oscillation of the fields. In vacuum, each wave propagates at a speed, c, of 2.997 x 108 m/s, independent of the frequency of the wave. As with any wave, the distance the wave travels in a single oscillation, the wavelength λ, the frequency f, and the speed of the wave c, are related by the simple equation c = λf . The hues which we perceive as visible light are but a small portion of the total light emitted by a star. Violet light has the shortest wavelength, about 400 nm, and red light the longest, about 700 nm. Ultraviolet (UV) light has shorter wavelengths and infrared (IR) light longer wavelengths, ranging out to 10,000 nm. All incandescent bodies have in common that their light is composed of a range of colors spreading from the infrared through the visible and on into the ultraviolet spectrum, as indicated in the figure at right. The relative intensity is always higher in the midrange than on the extreme ends of the spectrum. The position of the maximum relative intensity is inversely proportional to the absolute temperature, T, of the incandescent body. The total intensity, represented by the area under the curve, is proportional to the fourth power of the absolute temperature, however. The continuous nature of the spectral distribution is a consequence of the fact that a normal incandescent object composed of many different materials. The radiation emitted by a pure chemical substance, whether an element or a compound, differs in an important way from the radiation from a normal incandescent object. For a pure substance, the light spectrum is no longer continuous. Rather, it is said to be discrete. It consists of light of only a few, widely spaced spectral components. Moreover, the particular colors or components which are present are characteristic of the material which emits them. As in the case of the normal incandescent object, the intensities vary as a function of the wavelength, as shown in the figure at right. The intensities of the individual colors (or spectral lines) depend not only on the absolute temperature, but on characteristics of the material as well. The distinction between continuous and discrete spectra has practical importance for energy conservation. A normal incandescent light not only emits strongly in the visible region of the spectrum, it emits substantial amounts of radiation in the infrared region as well. This is perceived as heat. A fluorescent light, on the other hand, is designed to work by causing emission of light from just a few chemical substances chosen so that the spectral components which are emitted are located primarily in the visible region. The fluorescent light, then, is more efficient since a relatively greater amount of the energy used to operate the bulb appears in the useful part of the spectrum. 2. Procedure A spectrometer will be used to separate in space the spectral components which make up the emissions from a given light source, and to record information about the wavelengths of these components. In a spectrometer, a device such as a prism or a diffraction grating is used to send spectral components of different wavelengths off in different directions. Since diffraction gratings disperse the spectral components more widely than a prism and so make the components easier to see, one will be used in this experiment. A diffraction grating is constructed by ruling a series of closely spaced, parallel lines on a suitable surface. When the grating is illuminated, the lines act as secondary sources of light, and the secondary wavelets can interfere with each other when they come together at a distance. The wavelets can enhance or diminish each other. Which of these occurs at a given point depends on the distances followed by the waves as they propagate from the source, through the grating, to the point of observation. If the paths differ by an integral number of wavelengths, a condition called constructive interference occurs, and the two waves enhance each other. The original single beam is separated into several beams, as shown in the sketch at left. If the beam is monochromatic, i.e. has a single wavelength, λ, then the angles, θm, at which the beams appear is given by the formula mλ = d sinθm . Here d is the distance between adjacent lines on the grating, and the index m is called the order of the beam. Note that the direction of the incident beam is taken as the reference direction. The diffracted beam in that same direction is called the central beam and, consistent with the diffraction formula, is considered to be of order m = 0. For a gaseous chemical element, each of the spectral components will be diffracted. The central beams of each pattern will coincide, and that light will have the same appearance as the original light. As the diffraction formula shows, the angle at which a beam of wavelength appears in a given order m depends on λ. The angle θ1 for violet light will be about half that for red light since the wavelength of the violet light is about half that of the red light. A viewer looking back toward the source will see a series of bright lines of different colors as the position of observation shifts from one side to the other. If the grating space d is small, the dispersion of the beam will be great and the separate colored spectral components will be clearly distinguishable. The spectrometer uses this dispersion of the beams to permit making precise measurements of the angles at which the beams appear. The diffraction grating is mounted on a table at the center of the instrument and is illuminated by an appropriate source. An eyepiece is mounted on a movable arm which is then rotated to a viewing position where a given spectral component is visible. An indicator on the arm marks the position on a calibrated scale of angles fixed to the instrument. The scale is calibrated to 0.5°, or 30 min (30’) of arc. A vernier scale is often also available which permits determining changes of arc to within 1 min., but this refinement will not be used here. The typical discharge tube will provide a line of polychromatic light. The spectrometer is aligned with the arm in the center position and the light source opposite the eyepiece. The diffraction grating is mounted on the support table, perpendicular to the line of sight. The movable arm is rotated until the central beam appears at the cross hair of the eyepiece. The position of the arm should be noted as the reference position. The angles θm associated with the order m are found by rotating the arm to the right or to the left until the hair line rests on the line. An average of the two readings is taken to minimize any residual error in the initial alignment. Calibration of the Grating Spacing: The grating spacing is often known from other information. However, it is possible to measure it directly using light of a known wavelength. Here the green light from mercury (Hg) will be used. Its wavelength has been measured to be 546.1 nm. The procedure will be to determine the average θm for two or three orders, and to calculate the spacing from the diffraction formula for each separate order, and then finally to average the different values of d to obtain the working value. Hydrogen Spectra: A discharge tube containing hydrogen produces the light of atomic hydrogen. This light is rich in spectral lines which can be described by a simple formula. The formula, an empirical relationship; was first found in the previous century by Rydberg. It was first derived theoretically in 1913 by Neils Bohr. An atom of hydrogen consists of a proton and an electron which are bound to each other through the attraction of the electric force. In contrast to what might be expected from macroscopic phenomena, at the scale of atoms, not all possible energies are available to the electron. Rather only certain energy levels are available to the electron. According to quantum mechanics, with which Bohr’s theory is consistent, these levels are given by the formula En = -(2.18 x 10-18J)/n2, n = 1, 2, 3, … Light is emitted from the atom when the electron experiences a quantum jump from an initial level, n i, to a final level, nf. The change in energy of the electron is made apparent by the emission of a quantum of light, a photon, which has a frequency given by the difference between the energies of the two levels, divided by Planck’s constant, h. (h has the value 6.626 x 10 –34J – s) f = c/λ = (Ei – Ef)/h . It follows from these expressions that the inverse wavelength, 1/λ. is given by 1/λ = R(1/n2f – 1/n2i), where R, the Rydberg constant, has the value 1.0974 x 10 7m-1 . The light emitted when the electron reaches the lowest energy, with nf equal to 1, is in the UV, and cannot be seen. The light emitted when the electron winds up on the second lowest level, with nf equal to 2, contains light in the visible part of the spectrum. It will be investigated here. The light emitted when electrons finish in levels with nf equal to 3 or more is in the IR, and also is not visible. It is possible to verify the presence of the invisible UV or IR light with other types of detectors. The visible light is called collectively the Balmer series. It begins with a red light, for n i equal to 3, and goes on to other colors, with successively higher values of ni. The light source used with the spectrometer should be changed to a hydrogen discharge tube, and the instrument realigned. The angle, left and right, should be observed for each of the visible spectral components in the visible spectrum in the first two orders, m equal to 1 and 2. The diffraction formula should then be used to calculate a value of λ for each of the separate colors, and the index ni consistent with that wavelength determined. The table below should be used to organize the data and to complete the calculation. The experiment will be completed by observing the spectrum of an incandescent lamp. Physics 111: Elementary Physics Pre-Lab Exercise Atomic Spectra Name: ______________________ Section: _____ Calibration of the Grating Spacing: A group of students has taken the following data by observing the green line of mercury using a spectrometer. Find the spacing of the diffraction grating n the spectrometer. order(m) θright (deg) θleft (deg) θav (deg) d 1 19.10 19.13 _______ _______ 2 40.90 40.94 _______ _______ dav _______ Hydrogen Spectrum: Use the sample data below, taken with a hydrogen lamp, to complete the data table. color order violet violet 1 2 θright (deg) 15.08 31.40 θleft (deg) θav (deg) 15.12 31.35 _______ _______ λ ______ ______ λav blue blue 1 2 17.00 35.68 16.94 35.72 _______ _______ 1 2 23.22 51.95 23.17 51.90 _________ ________ _______ _______ λav red red n1 _______ _______ _________ ________ _______ _______ λav _________ ________ Questions: 1. State briefly (100 words or less) the purpose of this experiment . 2. Begin with the Bohr expression for the energy of the electron in level n, and provide the explicit details needed to derive the Rydberg expression and the value for the Rydberg constant. 3. The wavelengths of the visible lines of the hydrogen spectrum have been measured in terms of the wavelength of a particular line of the Hg spectrum. The wavelength of that line was given to four significant figures. As a result of accumulated errors in determining the grating spacing d and the angles at which the hydrogen lines were observed, how many significant figures do you estimate are you entitled to quote for the hydrogenic wavelengths ? Physics 111: Elementary Physics Lab Report Atomic Spectra Investigators: ________________________ , ________________________ , _______________________ _______________________ ________________________ Date: _____________ Procedure: Describe briefly (200 words or less) the procedures used in this experiment. Data: Calibration of the Grating Spectrum: order(m) θright (deg) θleft (deg) θav (deg) d _______ ________ _______ _______ _______ _______ ________ _______ _______ _______ _______ ________ _______ _______ _______ dav _______ Hydrogen Spectrum: color order θright (deg) θleft (deg) θav (deg) _____ _____ ____ ____ ________ ________ _______ _______ _______ _______ λ ______ ______ λav _____ _____ ____ ____ ________ ________ _______ _______ _______ _______ ____ ____ ________ ________ _______ _______ _________ ________ ______ ______ λav _____ _____ n1 _______ _______ _________ ________ ______ ______ λav _________ ________ Incandescent Lamp: Complete the experimentation by replacing the hydrogen discharge lamp with an incandescent lamp. Observe the spectrum, and estimate the upper and lower bounds of the visible spectrum, as seen by eye. order θright (deg) θleft (deg) θav (deg) red _______ _________ ________ _______ _______ violet _______ _________ ________ _______ _______ color Discussion: Summarize briefly the results which you have observed. λ