Amylase Teacher`s Notes pH
advertisement

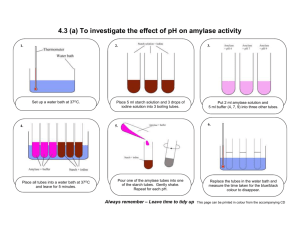
4.3 (a) To investigate the effect of pH on amylase activity Teacher Notes Apparatus required per class group of 24 students Enzyme source eg. Amylase Starch Range of buffer solutions Ph paper Water bath 1 Boiling tubes 60 Thermometer 12 Disposable gloves Labels 36 Timer 12 Dropper 12 Boiling tube rack 12 Boiling tube holder 12 The apparatus for this experiment lends itself to being pre-prepared in a ‘box of equipment’ – see Section 1. Advance preparation o Prepare solutions o Set up water bath at 370C Advance chemical preparation (a) Starch solution: o Shake 1 g of soluble starch (Analar) on to100 ml of water and bring to boiling point to obtain a clear solution. Cool. o Make up to100 ml of water. o Starch solution should be freshly prepared every time it is used. Biology SLSS 2009 1 (b) Amylase solution o Add 1g of amylase solution to a little distilled water. o Make up to 100 ml with distilled water. Safety precautions o See MSDS for further information Expected outcome of experiment o The enzyme works best at a pH of 7. o At this pH, the blue black colour will fade the quickest. Disposal and post-experiment work o Amylase – mix with excess sand (100 g: 5 L) and put in refuse. Flush solutions with excess water to foul drain o Buffers – dissolve small quantities (50 g) in 5 L of water and flush to foul water drain. Biology SLSS 2009 2 4.3 (a) To investigate the effect of pH on amylase activity Student Notes Apparatus required per group o Amylase solution o Starch solution o Range of Buffer solutions o pH paper o 6 x Boiling tubes o 1 x Thermometer o Labels o 1 x Timer o 1 x Boiling tube holder o 1 x Boiling tube rack Assembled apparatus Biology SLSS 2009 3 Method 1. Set up a water bath at 37OC. 2. Into each of 3 boiling tubes put 5 ml starch solution and 3 drops of iodine (each will go blue / black in colour). 3. Into each of 3 more boiling tubes put 2 ml of amylase solution. 4. To each amylase solution add 5 ml of one of the buffer solutions. Label each tube with the appropriate buffer, e.g. pH 4, 7, 9 etc. 5. Place all the tubes into the water bath at 37OC and leave for 5 minutes. 6. For each pH pour the amylase into the starch tube. Gently shake the tube to mix the contents. 7. Replace the tubes in the water bath and measure the time taken for the solution to go colourless – the blue/black colour will fade as the amylase changes the starch into sugar. 8. Test for sugar at the end by adding Benedict’s solution to the boiling tube and heating for 5 minutes – a brick red colour indicates the presence of sugar. 9. Repeat the entire experiment with boiled amylase. This will act as a control. 10. Draw a graph of your results. 11. At the end of the experiment, clean all of the equipment and replace it in its correct place. Results: pH of buffer Time taken for solution colour to change Conclusion/Comment Biology SLSS 2009 4