Co precipitation Synthesis, Physical and Magnetic Properties of Zinc
advertisement

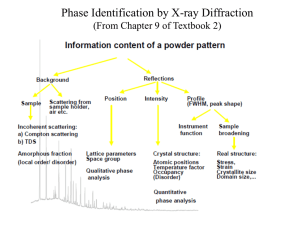
World Journal of Engineering Co precipitation Synthesis of Manganese Ferrite Powder Rabab Zahira, Ghiyas Anwar, Irfan Elahi, Kiran Mehmood and Arifa Jamil Department of Physics, University of Agriculture, Faisalabad, Pakistan In the present work, ultra-fine, nano-sized, magnetic Manganese ferrite powder has been prepared by coprecipitation technique using metallic chlorides of Mn and Fe as precursors and studied by X ray diffraction (λ=1.5404Ǻ) and Scanning Electron Microscope. 2. Experimental Procedure Nano-sized powder of magnetic Manganese ferrite was prepared by coprecipitation method using Manganese Chloride, Ferric Chloride Hexa-hydrated and Sodium Hydroxide as starting materials. The ratio between Fe and Mn was kept at 2:1 respectively. A 100 ml of salt solution was added drop by drop to the base solution until the Me/OH (metallic to hydroxyl ion) ratio reached to 0.2. The gray precipitates were obtained in this process. Then the beaker containing precipitate solution was placed in preheated water bath at 95°C for 5 hours. The obtained product was washed and filtered repeatedly with distilled water until the pH of the residual solution reaches 7. This process took about 6 to 7 hours. The sample was then dried in an oven keeping temperature at 50°C for 3 hours. After attaining the powder form by motor and pestle, calcinations process was carried out at 600°C for 4 hours to get the final product of Manganese Ferrite. This final Manganese ferrite powder was characterized by X ray diffraction, Scanning Electron Microscope and Vibrating Sample Magnetometer. The particle size was calculated by Sherrer’s formula given below, from the full width at half- Abstract Magnetic Manganese Ferrite Powder was synthesized via chemical co-precipitation, a highly fine way to produce chemically homogeneous powders with fine particle size, using metallic chlorides of Manganese and Iron. Sodium Hydroxide (NaOH) base was used as precipitant agent. The calcinations were performed at 600°C for 4 hours. The structural investigation of the prepared sample was performed with X-Ray Diffraction method. The crystallite size was 26.50nm, calculated using Sherrer formula. Key words: ferrite, Co precipitation, XRD 1. Introduction Due to their diverse and fascinating applications in wide technological and scientific fields, the synthesis and characterization of nanosized magnetic materials have emerged as an important subject during last several years [1-3]. Among these, ferrites, ferromagnetic having cubic spinel structure (MFe2O4) are complex versatile materials have been extensively investigated by the scientists in view of their unique optical, electrical and magnetic properties [4-6]. Several Physical and Chemical methods like, Hydrothermal [7-9], Co-precipitation [10-11], Combustion [12], Sol-gel [13] and some other techniques are being employed for the synthesis of these nano-sized spinel ferrites.Manganese ferrite powder is exceptionally important member of ferrite family with a variety of applications in modern era of science and engineering. 1441 World Journal of Engineering maximum of the XRD pattern for the (311) plane of the spinel structure. t = (0.9 λ / B) cos ӨB Here t is the mean crystallite size, B is the half width of the relevant diffraction peak, λ the x-ray wavelength and θ is the angle of diffraction [14-15]. The broadening (B) of the diffraction peak corresponds to the size of the crystal. 3. Results and Discussion The XRD pattern recorded for the magnetic ferrite powder obtained by coprecipitation and calcined at 600°C is shown in figure 1. The pattern of powder exhibited all the major peaks related to single phase cubic spinel structure of Manganese Ferrite. No external peak was detected in the prepared sample. The lattice parameter calculated from XRD information is 8.489Ǻ. The particle size was measured by Scherer’s formula using peak of maximum intensity of (311) plane. The value of particle size is calculated as 23.50 nm. The values of lattice constant and the particle size are in line with the previous published results of Manganese Ferrite prepared by coprecipitation as well as other techniques. References [1] D.D. Awschalom, D.P. DiVicenzo, Phys. Today 4 (1995) 43. [2] K. Raj, R. Moskowitz, J. Magn. Magn. Mater. 85 (1990) 233. [3] R.F. Ziolo, E.P. Giannelis, B.A. Weinstein, M.P. O’Horo, B.N. Ganguly, V. Mehrotra, M.W. Russel,D.R. Huffman, Science 257 (1992) 219 [4] A.T. Ngo, M.P. Pileni, J. Phys. Chem. B 105 (2001) 53. [5] S. Singhal, J. Singh, S.K. Barthwal, K. Chandra, J. Solid State Chem. 178 (2005) 3183 [6] B. Baruwati, R.K. Rana, S.V. Manorama, J.Appl. Phys. 101 (2007) 014302-221 [7] C. Hu, Z. Gao, X. Yang, J. Magn. Magn. Mater. 320 (2008) L70. [8] S. Verma, P.A. Joy, Y.B. Khollam, H.S. Potdar, S.B. Deshpande, Mater. Lett. 58 (2004) 1092 [9] O. Suwalka, R.K. Sharma, V. Sebastian, N. Lakshami, K. Venugopalan, J. Magn. Magn. Mater. 313 (2007) 198. [10] P. Mathur, A. Thakur, M. Singh, J. Magn. Magn. Mater. 320 (2008) 1364 [11] R. Aquino, J. Depeyrot, M. Sousa, F. Tourinho, E. Debois, R. Perzynski, Phys. Rev. B 72 (2005) 184435 [12] C.H. Yan, Z.G. Xu, F.X. Cheng, Z.M. Wang, L.D. Sun, C.S. Liao, J.T. Jia, Solid State Commun. 111 (1999) 287. [13] K. Mandal, S.P. Mandal, P. Agudo, M. Pal, Appl. Surf. Sci. 82 (2001) 386. [14] J. Nan, D. Han, M. Cui, M. Yang and L. Pan, J. of Hazard. Mater. 33(2006) 257 [15] B. D. Cullity, Elements of X-ray diffraction,Addison – Wesley Publishing Company, Canada, 1978 Fig.1. X ray Diffraction Pattern of Manganese Ferrite Powder 1442 World Journal of Engineering 1443