Freshwater Frenzy
advertisement

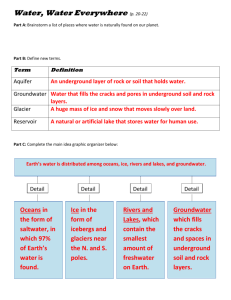
Freshwater Frenzy 6th Grade Created by: Abby Croucher, Misty Harger & Stephanie Hines Sponsored by: Indicators addressed: 6.3.8 Explain that freshwater, limited in supply and uneven in distribution, is essential for life and also for most industrial processes. Understand that this resource can be depleted or polluted, making it unavailable or unsuitable for life. 6.3.16 Explain that human activities, such as reducing the amount of forest cover, increasing the amount and variety of chemicals released into the atmosphere, and farming intensively, have changed the capacity of the environment to support some life forms. Objectives: Students will be able to Describe how freshwater is distributed around the world in different forms and different amounts Explain the difference between groundwater and freshwater and the different forms of each Define watershed Define acid rain and its sources Describe how acid rain affects wildlife Explain how different human activities pollute freshwater Materials Required: For Engagement activity: Where is the Water? o 9 clear, 1,000 ml containers (enough for groups of students, or as one demo) o Graduated cylinder o Masking tape o Marker o Tablespoon o Medicine dropper or pipette o Water supply For Centers: Exploring a Watershed model – this can be built beforehand by teacher or as a student project o Large plastic tub or storage container, e.g. rectangular under-the-bed containers o Aluminum foil or rocks of different sizes for creating watershed topography o Paper maiche (flour, water, newspaper, mixing bowl) or clear plastic to cover entire watershed o Water supply o Watering can o Map of local watershed (optional) WebQuest o Web Quest sheet (attached) o Access to the Internet Surface water & pollution study o Surface water photos and questions (attached) pH Basics o pH strips or pH meter o solutions for pH station – use a selection of 5-6 solutions of acids and bases lemon or lime juice--acidic any fruit juice—acidic soda pop--acidic baking soda in water--basic table salt in water--should be close to neutral, but may be slightly basic vinegar--acidic hand soap--basic any soaps/detergents--basic tap water--on a public system should be buffered around 7.3; if from a well could be different (posing a good question to students!) bleach--very basic**may be too hazardous (use your discretion) toilet bowl cleaner--very acidic**may be too hazardous (use your discretion) o plastic cups for solutions (one/solution) o Student sheet (attached) Design an Aquifer: o Plastic cups (one/student) o Gravel (one cup/student) o Sand (one cup/student) o Measuring cup o Water o Bucket for water waste o Old newspaper for drying out sand o Aquifer handout (attached) Science Journals Acid Rain Effects (to be assembled as a center and explored over the following week or so) o Vinegar o Water o 50 ml beakers o Large plastic cups with drainage holes poked in bottom or pots – 3 for each student or group o Radish seeds – 9 for each set of pots/cups o Potting soil o A sunny spot or grow light For Elaboration activity: Who Polluted the White River? See attached for materials. Lesson Introduction & Background Information: Water is a requirement for all life. Over 70% of the human body is water and over 70% of the earth’s surface is covered by water. The majority of the earth’s water (97%) is in the oceans; the remainder exists as freshwater. Seawater is unavailable for wildlife or human use, except in marine species and after desalination, a very costly, thus generally prohibitive, procedure. Therefore, life depends upon a very small amount of water. For humans, freshwater is a requirement in a number of areas. Most of the world’s freshwater is used for agriculture (70%). Industry takes up 22%, while domestic use requires the remaining 8%. However, throughout the different countries of the world, these numbers vary. In low income countries, such as those throughout Africa, Asia, South and Central America, an average of 87% of the freshwater is used for agriculture, leaving 8% for industry and 5% for domestic uses. In high income countries, such as Western Europe and North America, on average only 30% is required for agriculture, 59% for industry and 11% for domestic use. The proportion of use throughout the world varies because of population, infrastructure and economics, but also because of the uneven distribution of freshwater. For example, Algeria, a “water scarce” country, withdraws 142 m^3/capita/year; Niger, a “water stressed” country, withdraws 46 m^3/capita/year; and Sudan, with no threat to availability, 597 m^3/capita/year (http://www.worldwater.org/waterData.htm). Algeria and Sudan are similar in size and population, yet fall in arid-semi-arid and tropical zones, respectively. Niger is about half the size in land and population and falls in an arid zone (http://www.countrywatch.com/cw_regions.asp?REGION=1). Freshwater is divided into surface water (glaciers, lakes, rivers and streams) and groundwater (aquifers). Only a small percentage of the total freshwater is readily available for human use (.03% of the world’s total water amount). Most students are familiar with surface water, but few are familiar with groundwater. Groundwater Water that filters underground from precipitation or from surface water is groundwater. It moves below ground, downward due to gravity and horizontally, due to the tilt of the underground geology. It may also eventually seep into bodies of surface water (lakes, stream and oceans). Groundwater is held in the pores between rocks and soil particles. The size of the pores determines how much water is held and how quickly it flows. These pores may be saturated or unsaturated with water. The top level of saturated pores is the water table. The water table may fluctuate due to precipitation and groundwater extraction. http://ga.water.usgs.gov/edu/earthgw.html The storage mechanisms of groundwater are aquifers. Aquifers are underground geologic formations that can hold, transmit and yield water. Aquifers are generally made by confining layers (layers of material, such as clay, that are impermeable to other substances), by unconfined layers (such as silt, sand or gravel) that filter infiltrating water, or a combination of both. However, groundwater can become polluted by toxic substances, such as pesticides, gasoline additives, or other chemicals, that cannot be filtered by soil or that occur in such great quantities overwhelm the filtering capacity of soil. In such cases, groundwater is dangerous for human consumption. Another threat to groundwater is the depletion of aquifers, or groundwater mining. Humans extract groundwater by wells. Wells drilled into confined aquifers result in artesian wells because of the high pressure in the pressurized rock. Water can literally blast out without any aid of a pump. Groundwater is constantly restored by surface water infiltration or percolation from precipitation or surface water. However, when groundwater is withdrawn faster than it is restored, such as the case on a large scale in Texas and Arizona, aquifers become depleted. This can also happen on a smaller scale to individual homeowners whose wells “run dry.” The U.S. has a large supply of groundwater, holding 96% of its freshwater total (http://www.in.gov/idem/enviroed/lessonplans/water.html). Surface Water Surface water faces the same pollution risks as groundwater. Many human activities are having a negative affect on condition of surface waters throughout the world. Farming, deforestation, industry, vehicles, recreation, and lack of sewage systems all damage the cleanliness of surface water. While groundwater has the protection of filtering layers and time, the only protection between surface water and pollution are the human-created barriers. In many situations, these barriers simply do not exist. When surface waters become polluted, wildlife cannot be supported. Certain plants and animals can tolerate higher levels of pollution, but as pollution compounds, no wildlife can be supported. For example, a cyanide spill in Romania has killed everything, down to the bacteria, in the Tisza River. Waters through Hungary and Yugoslavia have slowly become infected, and the polluted waters now threaten Europe’s second largest river, the Danube (http://news.bbc.co.uk/1/hi/world/europe/642880.stm). In this lesson your students will be exploring surface water in a watershed model. A watershed is the land area that drains water to a common body of water (stream, river, lake or ocean). Watersheds are delineated by ridges and vary in size depending upon the topography. Watersheds vary in size and smaller watersheds are simply part of another, larger watershed. For example, the White River watershed is part of the Wabash watershed, which is part of the Ohio watershed, which is part of the Mississippi watershed. Acid Rain Your students will also explore one form of human-created pollution that affects water, acid rain. “Acid rain” (acids that fall out of the atmosphere) occurs in wet and dry forms. The wet form is fog, rain, or snow. The acidic water flows over and through the ground and affects a variety of plants and animals. The strength of the effects depend on many factors, including how acidic the water is, the chemistry and ability of the soil to resist change in pH, as well as the types of fish, trees, and other living things that rely on the water. The dry form refers to acidic gases and particles. About half of the acidity in the atmosphere falls back to earth through dry deposition. The wind blows these acidic particles and gases onto buildings, cars, homes, and trees. These gases and particles may also be washed from trees and other surfaces by precipitation, compounding acid to acid in the acid rain, making the combination more acidic than the falling rain alone. Wind is also a function in the affects of acid rain. The acid compounds are blown to places other than where the compounds were produced, spreading the disturbance. Acid rain results from the burning of fossil fuels. Coal, natural gas, and oil omit sulfur dioxide (SO2) (Sox) and nitrogen oxides (NOx) (Nox) when burned. The largest culprits in Sox and Nox are energy producers and vehicles. Cleaning up the omissions from power plants and vehicles and relying more on renewable energy (wind, solar and hydropower), are the best ways to reduce acid rain. http://www.epa.gov/airmarkets/acidrain/ Acid rain is measured by pH. pH is a logarithmic scale that runs from 1-14, growing less acidic (more alkaline) as the numbers increase. Pure water has a pH of 7; rain water has a pH of 5.5 (because of the carbon dioxide dissolved in it); acid rain in the US runs about 4.3 (http://www.epa.gov/airmarkets/acidrain/). In this activity, your students will explore the pH of different, common mixtures. They will make a non-toxic simulation of acid rain and will monitor its affect on plants. Procedures: 1. Engage your students in Where in the World is the Water? (adapted from Water, Water, Everywhere?, IDEM) a. Prepare before class 9 1,000 ml containers (beakers work best). Label them: ocean, icecaps/glaciers, groundwater, saline lakes, freshwater lakes, soil moisture, atmosphere & rivers. Label the 9th jar “All the water in the world” on one side; “Currently useable freshwater” on the other side. (You can also break students into groups so that each group has their own set.) Fill up the 9th beaker with 1,000 ml of water. b. Ask students to guess how the world’s water is divided into each of the categories. Place those amounts into the containers. Next demonstrate the true division (or print chart and hand out to individual groups to do): Source Amount in ml Oceans 973 Icecaps/glaciers 21 Groundwater (to 13,000 ft) 6.1 Saline lakes 0.08 Freshwater lakes 0.09 Soil moisture 0.05 Atmosphere 0.01 Rivers Can’t be measured http://ga.water.usgs.gov/edu/earthwherewater.html Percentage of Total 97 (approximate) 2.1 0.6 0.1 0.1 0.005 0.001 0.0001 The original beaker should now be empty. c. Ask students which of these are currently and realistically useable. d. Turn jar to reveal “Currently useable freshwater” and put back in it the groundwater, freshwater lakes, soil moisture, and river water. A total of 0.3% of the world’s water is useable by humans. e. Discuss with students what we use water for and compare to the amount of what is available. 2. Exploring freshwater. Before class, prepare several different centers in which your students can explore different forms of and issues regarding freshwater. Students need at least 10 minutes for each station. a. Design an aquifer b. WebQuest for surface water c. Exploring a watershed d. Surface water e. Learning about pH f. Acid Rain Effects g. Journaling 3. Discuss what the students discovered at each of stations. (See Background Information.) Go through any as a group, as necessary. 4. Continue to explore acid rain. Using the samples from the Acid Rain Effects Center, watch the affects of acid rain (in the form of vinegar) on seeds and plants. (Adapted from: http://web.stclair.k12.il.us/splashd/acradexp.htm) This is a good time to inform your students from the Background Information. 5. Bring together all of the activities in Who Polluted the White River? (Attached) Assessment Questions: 1. Explain the importance of conserving freshwater, and keeping it free of pollution. How is it even more important in certain areas? 2. If there was not any freshwater on Earth, how would that affect agriculture and industries? How would that affect life on Earth, including humans, plants, and animals? 3. Although about 75% of the earth is covered with water, explain why people throughout the world are focused on water pollution and conservation as an important issue. 4. Explain two human activities that have an impact on the whole environment. How do these activities impact wildlife? Extensions: Model the watershed on your local watershed using topographical maps. Use aquatic or terrestrial invertebrates instead of plants to monitor the affects of pH on wildlife. Learn about the factors that affect water quality and participate in a water quality monitoring. Go to http://www.in.gov/dnr/soilcons/riverwatch/ for more information. Make the pollution of the watershed into a WhoDunit? Connections: This lesson could have additional applications in Social Studies – including geography, economics, and history; chemistry; and made into a creative writing assignment.