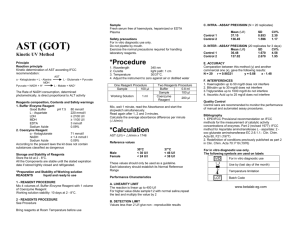
SOLUTION - Gravity Waves
advertisement

Experiment 9: A Chemistry Investigation Qualitative analysis in chemistry is very much like a detective game. The characters in a detective story have methods of operation and other distinguishing features. These characteristics make it possible to identify individuals as having been responsible for certain acts. The clues that one observes are evidence of some kind of interaction. In qualitative analysis you will make use of clues, evidence of chemical interaction, to help you identify the presence of specific ions in solution. However, before you can expect to identify the presence of ions, you must first become familiar with their characteristic behavior (method of operation). In this experiment you will be given four solutions, labeled 1, 2, 3, and 4. You will discover how they behave when they are mixed with four reagents labeled A, B, C and D. By making careful observations you will detect evidence of chemical reaction that will be characteristic for each of the solutions. These clues may be the formation of precipitates, change in color, production of a gas, production of heat or other evidence of chemical reaction. Recording your observations in tabular form will help recognize characteristic behavior for each of the four solutions. The solutions and reagents used in this experiment are deliberately identified by letter or number. This will permit you to focus your attention on the reasoning involved in developing an analytical scheme rather than on the actual reaction taking place. The scheme will permit you to identify unknown solutions according to their differentiating properties. Consider the following hypothetical case, reagents X, Y and Z, were allowed to react with a few drops of each of the solutions I, II, III and IV. The observations were recorded in the table as shown. SOLUTIONS/REAGENTS X Y I - + WHITE II + WHITE III + GASES IV - Z + YELLOW - + YELLOW - + YELLOW + WHITE - The + means distinctive evidence of reactions was observed and a - indicates no observable evidence or reaction. The color of precipitates or changes in color of solutions was also noted. If you were given an unknown solution which you were told was like one the four solutions tested, what method would you outline to identify the unknown? How many tests would you have to use? If another unknown gives a + test with X, is this sufficient evidence to identify it as one of the four solutions? Procedure 1. Obtain a set of solutions and reagents. Set aside a 400 mL beaker as a waste beaker. Page 9-1 2. Using the height of a 2mL sample of water in a test tube as a reference, place about 2mL of solutions 1, 2, 3 and 4 into a set of four clean test tubes and label them as 1, 2, 3 and 4. Add about 2 mL of reagent A to each test tube. Observe and record on the report sheet your results under the first column as + or – and include a brief description of any characteristic property of the + test. Pay attention to the formation of gases, precipitates, color changes or temperature changes of the mixtures. 3. Dispose the waste in the waste beaker, clean the test tubes. Place about 2mL of the solutions 1, 2, 3 and 4 to each test tube. Add about 2mL of reagent B to each test tube. Observe and record your results under the second column as + or – and include a description of any characteristic property of the + test. 4. Continue performing tests on each of the solutions 1, 2, 3, and 4 using reagents A, B, C and D until you have tested all possible combinations. Record your results as + or – and include a description of any characteristic property for the + tests under the appropriate column of your table. 5. Study the data carefully and note the identifying clues. Obtain an unknown from your instructor. Record your unknown number and test the unknown with reagents A, B, C and D to determine which solution it is like. Report your analysis together with a summary of the supporting evidence. Page 9-2 Chemistry Investigation Report Sheet SOLUTIONS/REAGENTS Name: _____________________ A B C D 1 2 3 4 SOLUTIONS/REAGENTS A B UNKNOWN# Which solution is your unknown? _________________________ Explain how you came to this conclusion. Page 9-3 C D Page 9-4 Chemicals for this lab: 15mL /student in dropper bottles (at least 3 of each) Label the following solutions as Solution 1 for 1.0 M sodium carbonate Solution 2 for 0.1M NaCl Solution 3 for 0.1 M barium chloride Solution 4 for 0.1 M lead(II) nitrate 15 mL/student in dropper bottles (at least 3 of each) Label the following reagents as Reagent A for 0.1M HCl Reagent B for 0.1 M silver nitrate Reagent C for 0.1 M sodium sulfate Reagent D for 0.1 M potassium iodate Unknowns: 10ml/ student from the solution 1,2,3 or 4. Please label them with unknown numbers. Page 9-5