Unknown Solutions Lab
advertisement

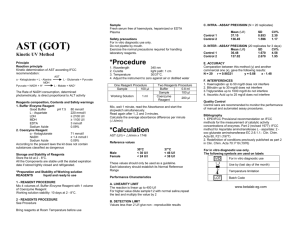
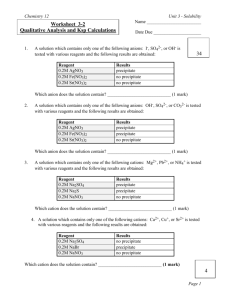
Name _____________________________________ Period ________ Date ______________ Partner’s Name ________________________________ Unknown Solutions Lab In this experiment you will observe the reactions that occur when four solutions are mixed with each of three different reagents. Using these reactions as a reference, you will develop a systematic set of tests to identify an unknown solution. The solutions and reagents are deliberately unlabeled; knowledge of the chemicals used is not important. Instead, focus your attention on making careful observations and developing a logical, systematic approach for identifying the unknown solution. Materials: Safety goggles Well-plate Solutions 1-4 Reagents A, B, & C Plastic wash bottle distilled water Safety: In this lab, the solutions you use may contain harmful materials. Treat all unknown solutions as if they were hazardous. Observe all precautions. Do not mix any chemicals except as directed in the Procedure section. Avoid skin contact with these chemicals. Procedure: 1. Clean the well-plate with distilled water. In separate wells, mix solutions 1 through 4, one at a time, with each of the reagents, A–C, following the grid on the Data Table. Use 4-5 drops of each solution. Make careful observations and record in your Data Table. A reaction occurs only if a precipitate (ppt) forms. Make sure you note the reaction as a ppt and the color of the precipitate in the data table. If no precipitate forms write No Reaction in your data table. 2. Do Question #2 on the lab sheet before emptying and cleaning your well plate. 3. Clean the well-plate with tap water and ask the teacher to check the station before leaving the lab. Analyses and Conclusions: 1. Based on your experimental results, how would you distinguish between (choose one reagent that shows a different reaction for each solution). You must tell which reagent was used and how each solution looked using that reagent. (You can use different reagents for each sub-problem): 1 & 3: __________________________________________________________________________ 2 & 3: _________________________________________________________________________ 1 & 2:__________________________________________________________________________ 1 & 4: _________________________________________________________________________ 2 & 4: _________________________________________________________________________ 3 & 4:__________________________________________________________________________ 2. Suppose someone has randomly relabeled the solutions used in this experiment as I, II, III, and IV and the reagents as X, Y, and Z. Using the facts that follow, consult the Data Table and correctly identify the solutions and reagents by their original designations (1, 2, 3, 4, A, B, C). When mixed: I and Y produce a red precipitate. II and Y produce no precipitate. II and X produce a yellow precipitate. III and Z produce a white precipitate. I = ___________ X = ___________ II = ___________ Y = ___________ III = ___________ Z = ___________ IV = ___________ Data Table: Results of Mixing Solutions and Reagents Solution 1 BaCl2 Solution 2 AgNO3 Solution 3 Pb(NO3)2 Solution 4 (NH4)2MoO4 Reagent A Reagent B Reagent C K2CrO4 HCl Na2HPO4 3. Write a balanced chemical equation for the reactions that occurred between solutions 1, 2 and 3 and reagents A, B and C. If no reaction occurred, write no reaction for the products. Balanced equations MUST include state symbols for the products!!! 1. BaCl2 (aq) + K2CrO4(aq) 2. BaCl2(aq) + 3. BaCl2(aq) + 4. AgNO3(aq) + 5. AgNO3(aq) + 6. Pb(NO3)2(aq) HCl(aq) Na2HPO4(aq) K2CrO4(aq) Na2HPO4(aq) + K2CrO4(aq)