Total Proteins Colorimetric Method
advertisement

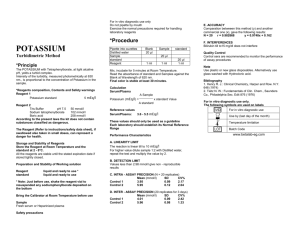
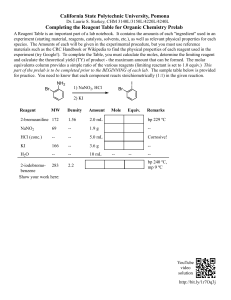
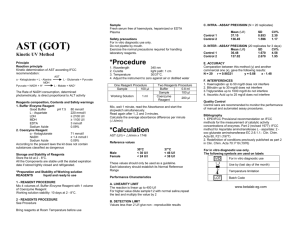
Safety precautions For in vitro diagnostic use only. Do not pipette by mouth. Exercise the normal precautions required for handling laboratory reagents. Total Proteins *Procedure Colorimetric Method Principle Serum Proteins, in alkaline solution and in presence of copper ions (Biuret Reagent), form a pink - coloured complex. Intensity of the colour is direct proportional to the Protein concentration Reagent Composition, Contents and Safety warnings R2. Reagent Sodium Potassium Tartrate Sodium Hydroxide Potassium Iodide Copper Sulphate R1. Standard Bovine Albumin Pipette into cuvettes Distilled water Sample Standard Reagent 2 Blank 20 µl Sample standard 20 µl 1 ml 1 ml 20 µl 1 ml Mix, incubate for 10 minutes at Room Temperature. Read the absorbance of Calibrator and Samples against the Blank at Wavelength of 540 nm. *Calculation 21 mmol/l 750 mmol/l 6 mmol/l 6 mmol/l 6 g/dl The Reagent is classified as C = Corrosive Content: SODIUM HYDROXIDE Risk: R34 Causes burn S26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advise S45 In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible) S61 Avoid release to the environment. Refer to special instructions/ safety data sheets S36/37/39 Wear suitable protective clothing, gloves and eye / face protection Storage and Stability of Reagents Store the Reagent at Room Temperature and the Calibrator at 2 - 8°C. All the components are stable until the stated expiration date if tightly closed. Preparation and Stability of Working solution Reagents: liquid and ready to use Bring reagents at Room Temperature before use. Sample Fresh serum free of haemolysis. Heparin or EDTA Plasma Comparation between this method (y) and another commercial one (x), gave the following results: N = 20 r = 0.98467 y = 0.99x + 0.20 F. INTERFERENCES Haemoglobin till to 50 mg/dl does not interfere Bilirubin till to 20 mg/dl does not interfere Quality Control Control sera are recommended to monitor the performance of manual and automated assay procedures. Bibliography 1. Henry R. J.: Clinical Chemistry, Hoeber, N.Y., 413 (1976) 2.Tietz N. W.: Fundamentals of Clin. Chem., Saunders Co., Philadelphia, PA 302 (1970) For in vitro diagnostic use only. The following symbols are used on labels For in vitro diagnostic use A Sample TOTAL PROTEIN (g/dl) = x standard Value A standard Reference Values 6.0 - 8.0 g/dl * * F. Pasquinelli: Diagnostica e Tecniche di Laboratorio; Vol.1, first part p. 666 (1979) These values should only be used as a guideline. Each laboratory should establish its Normal Reference Range Performance Characteristics A. LINEARITY LIMIT The reaction is linear till to 10 g/dl For higher value dilute sample 1:2 with distilled water, repeat the test and multiply the value by 2 B. DETECTION LIMIT Values less than 0.3 g/dl give non - reproducible results C. INTRA - ASSAY PRECISION (N = 20 replicates) Mean (g/dl) SD CV% Control 1 5.32 0.15 2.76 Control 2 4.81 0.11 2.23 D. INTER - ASSAY PRECISION (20 replicates for 3 days) Mean (g/dl) SD CV% Control 1 5.50 0.14 2.64 Control 2 4.81 0.13 2.73 E. ACCURACY Use by (last day of the month) Temperature limitation Batch Code