9-hydroxyfluorene (0.1822 g) and recycled HK-OMS
advertisement

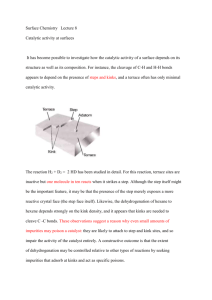
Rojas 1 Bethel College Creating a Greener Organic Chemistry Lab Jose A Rojas 05/19/2010 Chemistry 482 Senior Seminar Rojas 2 Table of Contents I. Abstract 3 II. Background 4 III. Methods and Experiments 7 a. Reagents 7 b. Synthesis of Catalyst 7 c. General Oxidation Reaction Procedures 8 d. Oxidizing Reactions Using K-OMS-2 8 e. Oxidizing Reactions Using H-K-OMS-2 9 f. 10 Oxidizing Reaction using recycled H-K-OMS-2 g. Oxidation using 0.4 g of H-K-OMS-2 by Organic Chemistry Class 11 h. Apparatus and Procedure 11 IV. Results 13 V. Discussion 14 VI. Conclusion 17 References 19 Acknowledgments 20 Appendix1 21 Rojas 3 I. Abstract The purpose of this project was to develop a green lab that could be used for a lab period for students in Organic Chemistry. The goals were to find a catalyst that was less toxic than ones currently in use for the oxidations of alcohols to ketones and to complete the reaction and procedures needed for separation and purification within a three hour lab period. The lab developed is based on the oxidation of the alcohol 9-flurenol into the ketone form 9-flurenone. The catalyst originally used was a polymer-supported 𝐶𝑟𝑂3 , and the time for the completion of the reaction was an hour plus the time needed for all the other procedures needed to isolate the ketone. Octahedral molecular sieves (OMS) have shown a great potential for the oxidative catalysis of alcohols. These kinds of catalysts were investigated earlier by the Bethel graduate Omar Hasan in his seminar project last year. He studied a number of different OMS-2 type catalysts. However, in order to reach the goals of a green lab catalyst K-OMS-2 and H-K-OMS-2 were used because of their low toxicity levels. The results of this study indicated that the H-K-OMS-2 catalyst was able to complete the oxidation reaction within 20 minutes, while the K-OMS-2 catalyst did not work favorably toward goals. The lab developed in this project proved to be successful as demonstrated by the very good results obtained by the Organic Chemistry II class which recently tested the laboratory procedure. Rojas 4 II. Background Omar Hasan, a bethel grad, in his undergraduate research worked with different catalysts that enhanced the oxidation of alcohol into ketones and aldehydes. Hasan was working with OMS-2, a porous manganese oxide octahedral molecular sieve. It is called OMS-2 because it has a 2x2 octahedron crystalline structure. Hasan indicated that the porosity of the OMS-2 give it the ability to channel some of the positive charges for superior catalytic activity. Figure 1, shows the Figure 1. Structure of OMS-2. structure of OMS-2. The OMS-2 channels have porous openings of 4.6Å. The OMS-2 structures are comprised of units of 𝑀𝑛𝑂6 octahedron. OMS-2 consists of manganese oxide, 𝑀𝑛𝑂2 , that share corners and edges that line up forming the 𝑀𝑛𝑂6 octahedron. Even though, OMS-2 serves as a highly active thermally stable catalyst when ion exchanged with other metal ions, for example, vanadium and nickel, we will focus on the original form of Figure 2, Proposed Mechanism for Catalyzed Alcohol Oxidation by OMS-21. OMS-2 (K-OMS-2) and on the hydrogen doped form of the OMS-2 (H-K-OMS-2). The hydrogen doped form is achieved by washing the K-OMS-2 with 1.0 M 𝐻𝑁𝑂3 . By doping OMS-2, Hasan indicated that this significantly enhanced the conversion of alcohols to either the ketone or aldehydes form, which Rojas 5 suggested that the oxidation was enhanced by Bronsted acid sites1. In figure 3, there is a list of the conversion of several alcohols and their conversion percentages when using the acid doped form of OMS-2, as well as, the original form K-OMS-2. The proposed oxidation mechanism for these oxidation reactions is illustrated in figure 2. Hasan also mentioned that H-OMS-2 and KOMS-2 apart from being very stable catalyst that they can be store and be active for up to two years. Hasan found that these oxidizing reactions ended up Figure 3. Oxidation of Alcohols Using K-OMS-2 and H-K-OMS-2.1 as liquid products which eventually were analyzed by gas chromatography taking a very long time; this technique was use for qualitative purposes. The search for greener catalysts that can oxidize reactions is becoming more and more popular today. In experiment 14 of the book Green Organic Chemistry2, the focus is on oxidation chemistry, where a secondary alcohol, 9-flurenol, is oxidized into its ketone form, 9-fluorenone, by using a polymer containing a reactive form of 𝐶𝑟𝑂3 as their oxidizing agent. Figure 4 shows the reaction. The polymersupported 𝐶𝑟𝑂3 oxidizes organic substrates very readily. In the introduction to the lab, it mentions that typical oxidizing agents are often corrosive, toxic, and environmentally damaging, and that the development of environmentally benign procedures for the adjustment of oxidation state remain an Rojas 6 important research goal2. In their lab, the experiment calls for refluxing the reactions, thin-layer chromatography (TLC) for checking the progress of the reaction as well as for qualitative and quantitative analysis, rotary evaporation of the Figure 4. Oxidation reaction from 9-fluorenol to 9-flurenone solvent and the recrystallization and melting point determination for qualitative analysis. The procedure used 1 gram of the alcohol and 5 g of the dry polymer-supported 𝐶𝑟𝑂3 catalyst to 35 ml of toluene in a 100 ml round bottom flask containing a magnetic bar. It was refluxed for about an hour while stirring and the reaction was check by TLC on silica plates for completion. Reaction was cooled to room temperature, filtered to remove the catalyst, removed the solvent with a rotary evaporator and weighted the crude product. Finally, recrystallyzed the crude product with a mixture of ethanol and water, weighted the recrystallized product and took the product’s melting point. The catalyst can be treated and stored ready to be reused. Green chemistry is becoming the most popular way of doing chemistry. In order to do Green Chemistry, one or more of the twelve principles of green chemistry need to be follow when doing an experiment. For example, in order to achieve the goal of creating a greener lab some of these principles had to be followed. The followings are green chemistry principles provided by the Environmental Protection Agengy (EPA) website that were followed through this project: preventing waste, less hazardous chemical syntheses, designing safer chemicals, design for energy efficiency, catalysis and realtime analysis for pollution prevention. By combining the OMS-2 catalysts that Hasan studied with following the procedures and using the reagents that were used in experiment 14, in the book of Green Organic Chemistry, the goal for this project to develop a “Green Lab”. The reaction needed to be completed in less than a three hour period, so that this lab could be used during a lab period for the Organic Chemistry class in Bethel College. This project focused on using Hasan’s greenest catalyst which Rojas 7 includes the doped catalyst H-K-OMS-2 and the original form K-OMS-2. The oxidation reaction of 9fluorenol to 9-fluorenone is expected to work since Hasan was able to get very good conversion percentages when oxidizing phenyl ethanol, as well, as benzhydrol with the doped OMS-2 form H-KOMS-2 and K-OMS-2. It should be a noted that Hasan used a vanadium\𝑀𝑛𝑂2 catalyst which in most cases had very high conversion percentages. However, for our purposes of a green lab, this form of catalyst will not work since vanadium is very toxic. In the experiment 14, they used a polymersupported 𝐶𝑟𝑂3 as their catalyst. 𝐶𝑟𝑂3 itself is much more toxic than our manganese oxide octahedral sieves which help us achieved our goal of a green lab. Lastly, the catalyst use for this project should be tested to see if it can be recycled or not in order to favor our purposes to create a green lab. III. Methods and Experiments A. Reagents All reagents used were analytical grade and purchased from SIGMA-ALDERICH chemical supplier. Ultrapure deionized water (UDW) was used to prepare materials. Water was purified by a compact ultrapure water system by Barnstead. B. Synthesis of Catalysts 1. K-OMS-2 4.3484 g. of 𝐾𝑀𝑛𝑂4 was placed in a 100 ml beaker and dissolve in 75 ml UDW. 6.5272 g. of 𝑀𝑛𝑆𝑂4 . 𝐻2 𝑂 was placed in a 250 ml round bottom flask and dissolved in 22.5 ml UDW along with 2.3 ml concentrated 𝐻𝑁𝑂3 . Then the initial solution of 𝐾𝑀𝑛𝑂4 was carefully added to the 𝑀𝑛𝑆𝑂4 . 𝐻2 𝑂 solution. From this mixture a very dark mud like precipitate was precipitated. This mixture was reflux at about 100℃ for 24 hours. The product was vacuum filtered using a paper filter, washed with 7 small portions of UDW totaling 100 ml and dried at 120℃ overnight in an oven. After drying, the product was separated from the filter paper and ground to a powder by Rojas 8 mortar and pestle. The product was then stored in a sealed glass vile. The final yield was 6.1140 g. of K-OMS-2. 2. H-K-OMS-2 0.9973 of the K-OMS-2 was exchanged with 200 ml of 1.0 M 𝐻𝑁𝑂3 . This was done with vigorous stirring at room temperature for 2 hours. The product was then filtered by vacuum filtration and a paper filter. The product was vacuum filtered using a paper filter, washed with 7 small portions of UDW totaling 100 ml and dried at 120℃ overnight in an oven. After drying, the product was separated from the filter paper and ground to a powder by mortar and pestle. The product was then stored in a sealed glass vile. The final yield was 0.9284 g. of H-K-OMS-2. C. General Oxidation Reaction Procedures 9-hydroxyfluorene (0.182 g.) and K-OMS-2 catalyst (0.4 g.) were refluxed with toluene (10 ml) in a round-bottom-flask (100 ml) containing a magnetic stir bar for 20 minutes. The reaction was followed every ten minutes by thin layer chromatography (TLC) on silica plates, developed with 30% acetone in hexanes. The reaction was cooled to room temperature; catalyst was removed by gravity filtration and washed with small amounts of toluene. Catalyst was dried and stored for future possible use of it. The solvent was removed by rotary evaporatorion. Small stream of air used to remove the last traces of the solvent. The crude product was recrystallized from a mixture of 50:50 ethanol/water and melting point determined. D. Oxidation reactions using K-OMS-2 1. Experiment #1 9-hydroxyfluorene (0.1824 g) and K-OMS-2 (0.505 g) catalyst were added. The reaction was refluxed for about an hour and a half. The reaction was not completed after this time when it Rojas 9 was checked by TLC. The recrystallize product was a white and yellow precipitate, nothing like our desire product, probably a mixture of starting materials and product. 2. Experiment # 2 9-hydroxyfluorene (0.1824 g) and K-OMS-2 (0.501 g) catalyst were added. The reaction was refluxed for about an hour and a half. The reaction was not completed after this time when it was checked by TLC. The recrystallize product was a white and yellow precipitate, nothing like our desire product, probably a mixture of starting materials and product. 3. Experiment #3 (using more catalyst) 9-hydroxyfluorene (0.182 g) and K-OMS-2 (0.2 g) catalyst were added. The progress of the reaction was followed every ten minutes by thin layer chromatography (TLC) on silica plates, eluting with 30% acetone in hexanes. The reaction never went to completion after the hour and a half, it seemed like the reaction was still going, and the spots in the silica plates looked like if there was a 50:50 mixture of starting material and product. E. Oxidizing Reactions using H-K-OMS-2 1. Experiment #1 9-hydroxyfluorene (0.1823 g) and H-K-OMS-2 catalyst (0.05 g.) were added. The reaction was refluxed and stirred for about 40 minutes. After 40 minutes, the solvent was evaporated and we were unable to continue with the experiment. 2. Experiment #2 9-hydroxyfluorene (0.1827 g) and H-K-OMS-2 catalyst (0.0505 g.) were added. The reaction was not complete when checked with TLC after an hour and a half of refluxing. The recrystallized product was a light yellow precipitate with a melting point range of 104-118 ℃. The wide melting point range indicates impurity and that it is probably a mixture of starting material and product. The recrystallized product weighted 0.0737 g. with a percent yield of 40.79%. Rojas 10 3. Experiment #3 (using more catalyst) 9-hydroxyfluorene (0.1824 g) and H-K-OMS-2 catalyst (0.2 g.) were added. The reaction was complete after an hour and a half and the solvent had a yellow color. The recristallized product was very fine yellow crystals, as expected and its melting point range was 78.5-82 ℃ . The melting point range was within ±3℃ of the melting point of the desired product, which means that the product is pure. 4. Experiment #4 (even more catalyst) 9-hydroxyfluorene (0.1830 g) and H-K-OMS-2 catalyst (0.4074 g.) were added. The reaction was complete after 20 minutes. The progress of the reaction was checked every 5 minutes for completion. The solvent was of a yellow, a good indication. The recrystallized product was yellow crystals, and its melting point range was 81.5-84 ℃ . The recrystallized product weighted 0.0245 g. with a percent yield of 13.56%. 5. Experiment #5 9-hydroxyfluorene (0.1827 g) and H-K-OMS-2 catalyst (0.405 g.) were added. Reaction complete after 20 minutes. The product was yellow crystals, melting point 79.5-83.5 ℃ . 6. Experiment #6 9-hydroxyfluorene (0.1833 g) and H-K-OMS-2 catalyst (0.4022 g.) were added. The reaction was complete after 15 minutes. The product was yellow crystals, melting point 79-83 ℃ . 7. Experiment #7 9-hydroxyfluorene (0.1836 g) and H-K-OMS-2 catalyst (0.4048 g.) were added. Reaction complete after 20 minutes. The product was yellow crystals, melting point 81.5-84.3 ℃ . 8. Experiment #8 9-hydroxyfluorene (0.1834 g) and H-K-OMS-2 catalyst (0.4015 g.) were added. Reaction complete after 20 minutes. The product was yellow crystals, melting point 80.5-83.5 ℃ . Rojas 11 F. Oxidation using recycled H-K-OMS-2 1. Experiment #1 9-hydroxyfluorene (0.1822 g) and recycled H-K-OMS-2 catalyst (0.4033 g.) were added. The reaction was refluxed for about an hour and a half. The progress of the reaction was followed every ten minutes by TLC. It seems like the reaction never went to completion, this could be cause by the catalyst being completely doped from previous reactions. G. Oxidation using 0.4 g of H-K-OMS-2 by Organic Chemistry Class The organic chemistry class was able to run this experiment in order to see if, in fact, this lab could work as a lab period for future Organic Chemistry courses. The organic chemistry class ran seven reactions using the same procedures in all of them. The procedure that the class followed is shown in appendix 1. H. Apparatus and Procedure The synthesis of the catalyst as well as the oxidation of the alcohol had the same set up. The apparatus shown below (image 1) was set up so that two reactions could be running at the same time; by doing this we saved water as well as time. The round bottom flask was loaded with Image 1, Set up used in synthesizing catalysts and oxidation reactions Condenser Round Bottom Flask Heating Mantle Stirrer Drain Water Source Temperature Control Rojas 12 reactants in order for the reaction to proceed. In all the oxidation experiments, a 100 round bottom flask was use, and a 250 ml was use for the synthesis of the catalyst. Once the reactions were completed, the catalyst was dried and grounded to a powder by mortar in order to be a) b) zero time 10 min 20 min Image 2a, shows the TLC silica plates already spotted at zero time, ten minutes and 20 minutes after reaction began. Image 2b, shows the same silica plates under UV light, where we can see the spots. use. The oxidation reactions were analyzed by TLC, image 2 shows the TLC set up that was use to check the progress of the reaction as well as its completion. The silica plates were spotted with the starting material dissolve in toluene, the reaction, and the desire product dissolve in toluene. The reaction spot was compared to that of the starting material as well as the desire product. By doing this, we were able to analyze the reactions qualitatively and somewhat quantitatively. As mentioned before, the oxidizing reactions were cooled to room temperature, and then filtered before evaporating the solvent via rotary evaporator. Image 3, Electrothermanl melting point apparatus Rojas 13 Once we had our crude product, we recrystallized it and took its melting point using an electrothermal melting point apparatus (image 3). Taking the melting point is one of the most effective methods to check for the quality of the product. NMR was another option but we decided that taking the melting point took less time and a better method. IV. Results Since we could not find anything while using K-OMS-2 for our reactions, the results are based on the results that we have for the reactions with H-K-OMS-2 that were done by the Organic Chemistry class and me. Table 1. Oxidation Results using H-K-OMS-2 Experiments Experiment #1 Experiment #2 Experiment #3 Experiment #4 Experiment #5 Experiment #6 Experiment #7 Experiment #8 Amount of H-KOMS-2 0.05 0.05 0.2 0.4074 0.4 0.4022 0.4048 0.4015 Melting point range(°C) -104-118 78.5-82 81.5-84 79.5-83.5 79-83 81.5-84.3 80.5-83.5 Recrystillized product (g) -0.0737 -0.0245 Reaction Time -1.5 hr 1.5 hr 20 min 20 min 15 min 20 min 20 min % yield -40.79 --13.56 Table2. Oxidation Results using H-K-OMS-2 from the Organic Chemistry Class Experiments Experiment #1 Experiment #2 Experiment #3 Experiment #4 Experiment #5 Experiment #6 Experiment #7 Amount of H-KOMS-2 0.4 0.4 0.4 0.4 0.4 0.4 0.4 Melting point range(°C) 80-82.7 83.1-83.6 82-85 82-83 80-83 80-83 79.5-82.5 Recrystillized product (g) 0.059 0.02 0.078 0.09 0.09 0.34 0.01 Reaction Time 25 min 20 min 20 min 20 min 20 min 25 min 20 min % yield 32.65 11.07 43.17 49.81 49.81 188.2 5.53 Rojas 14 The results in table 1 were the ones that I recorded from my reactions. Notice that only seven of the eight reactions are on the table. The first experiment was not completed because the solvent dissolved after taking several samples from the solution, as I mentioned in the procedure segment for this particular experiment. From the results in table 1 and 2 we can see that after adding 0.4 grams of the H-K-OMS-2 catalyst, the reaction went to completion in an average of 20 minutes and with very good melting point ranges, by 80-83 ℃ being the melting point of 9-fluorenone. Most of the melting point ranges were within ±3℃ of our desire melting point range, which means that the final products were mostly pure. By looking at the results there is a greater confidence in that the H-K-OMS-2 catalyst works well for the oxidation reaction that we wanted, 9-flurenol to 9-flurenone. From the data we are confident to state that the reaction can be carry to completion within 20 minutes, this is a decrease in time from one hour to 20 minutes, saving 40 minutes of time that we can use for the following procedures in the lab. Also, from the data we are confident that the product is pure and that future reactions can obtain this purity by following the procedures stated well. Lastly, this lab proves that it can be repeatable by having several Organic Chemistry students doing it, while following the procedures provided, and getting the results that were expected in every case. V. Discussion There are many things to note in the results, as well as, in the procedures. First, there was not data collected from the experiments done with the K-OMS-2 catalyst since the reactions never went to completion after an hour and a half. One thing that could have been done is that the reaction could have been run for more time and be completed after three or four hours. Hasan’s oxidation reactions were run for four hours using different catalysts, and as mentioned before, he was able to Rojas 15 obtained favorable results using K-OMS-2 as well as H-K-OMS-2. But since my goal was to reduce the time that take for the reaction to be completed, different options had to be considered. One of the options was to increase the amount of catalyst but this did not work either. The reaction was still not complete after an hour and a half, which was the time limit for our purposes. When the melting point was taken from these different reactions using K-OMS-2 as the catalyst, there were broad melting point ranges, for example, in one there was a range of 104-118℃. This was a broad range which indicated impurity in our product. Also, the melting point of the starting material, 9-flurenol, is 153-154℃ and the melting point for the desire product, 9-flurenone, is 80-83℃; this melting point range were not even close to the starting material or the desire product. This indicated that the product was a mixture of starting material and product, and possibly some other kind of contamination. This is the reason why the results from K-OMS-2 were not presented, because they did not help towards achieving the goals for this project Another point to notice is that the results obtained while using H-K-OMS-2, as you can see in table 1, most of them did not include the mass after recrystallization, only the melting point ranges. This happened because the main thing was to prove that the product was our desired product. This is the reason why only the melting point ranges were taken, since this is a very good method in order to analyze sample qualitatively. Another reason is that when the reaction is filtered, the solvent is evaporated and the crude product recrystallized, there is a great amount of our product that is been lost in each of those procedures; so, the percent yield most of the times is very small. There were some instances that the percent yields were more than 100% which indicated that there was some kind of contamination of the product with the solvent that was not able to be evaporated. This happened to one of the persons in the organic chemistry lab, but this is normal when someone has not done these kinds of procedures before. Rojas 16 There is another point that should be noted. There was a great amount of confusion from the organic chemistry class and from myself about the amount of the mixture of ethanol and water used to recrystallized the crude product. From my part, at the beginning of my project I was uncertain about how much ethanol and water to use, the instructor indicated that I was supposed to use the least amount of ethanol and water as possible so that recrystallization could happen faster. After doing several recrystallizations, I was able to find a way to do it, even though I was not keeping track of the amount of ethanol and water that I was using. So, what I was doing was to dissolve the crude product in a small amount of hot ethanol, then pour a small amount of water until I could see the cloudy point, where a white precipitate is form, and then heat the solution and let the precipitate dissolve. After it was dissolve, I let the solution cool down and recrystallize. This technique was not a very good one in order to save time, but it worked. The organic chemistry students ran into similar problems, in the procedures that I provided I did not indicated how much ethanol or water to add which was somewhat confusing for some of them, but with the help of their instructor they were able to complete this lab almost perfectly. The amount of ethanol and water to use for recrystallization is the only thing that I could not find a good way to do it, but with the help of an instructor, someone can definitely follow this lab’s procedure and finish it within three hours, since this was one of my goals. Lastly, it has noted that the best catalyst for this experiment is the H-K-OMS-2, which is better in toxicity than the polymer-supported 𝐶𝑟𝑂3 used in the original procedure that was use as a base to this project. For this experiment, there was less amount of H-K-OMS-2 used compared to what they were using in the experiment that was been followed. They used 5 g. of polymer-supported 𝐶𝑟𝑂3 for every 1 g of 9-flurenol that they used. In our case, the amount of catalyst that we used was 0.4 g of H-K-OMS-2 for every 1 mmol (0.182g) of 9-flurenol. Also, the last thing that we should notice is that when we tried to use the recycled catalyst, the reaction never went to completion after an hour Rojas 17 and a half. This could be done to the fact that the “recycled” catalyst that was use was never reactivated, in other words, the catalyst was suppose to be wash with 1.0 M 𝐻𝑁𝑂3 which could have enable the catalyst to react again with 9-flurenol by being doped once again with the acid. This was never done and it would be a very interesting small project to do, where someone can run a couple of reactions following my procedure and recycling the catalyst. Eventually wash the catalyst with 1.0 M 𝐻𝑁𝑂3 to reactivate it and do a third reaction in order to see if the catalyst can, in fact, be recycled and reuse to favor our purposes for a greener lab. Hasan in his project indicated that it might be more expensive to try to recycle the catalyst and reuse it than making new catalyst. This could be true but in order to do new catalyst, there is a two day wait, a day where the catalyst is synthesis, and another to dry it and then two more hours for washing the catalyst with 1.0 M 𝐻𝑁𝑂3 . So, it might be worth trying to save and recycle the catalyst for future reactions. VI. Conclusion There are a few conclusions that can be drawn from this project. First, the goal of doing a greener lab was achieved by having the less toxic catalyst work very well for the desired reaction, which reacted in 40 minutes less than the catalyst originally used for this experiment. Secondly, the goal of putting together a lab that could work for future Organic Chemistry courses was achieved by having the present Organic Chemistry Class run the experiment and obtaining very good results, which indicated that this experiment can be reproducible and with very good results. The organic class was able to obtained pure products that were analyzed by melting point, a very good qualitative methods to use. Thirdly, the goal to be able to recycle the catalyst was not achieved, but further investigation needs to be done in order prove this. This is a project that someone in the future may perform and include to this project if the results come up to be favorable. Rojas 18 From the results that were obtained, there are possibilities that different organic compounds may be able to be oxidize while using this same procedure with the same catalyst. Hasan was able to oxidize different alcohols using this kind of catalyst, even though the procedures that he was following were different to the procedures followed during this project. This could be another project where someone could use this procedure while working with different alcohols. The best way to do these reactions is to do them as green as possible, and hopefully future students can be able to try and do a follow up in this project so that their findings can indicate us whether or not this procedure is good for a wide range of alcohols. Rojas 19 References 1. Hasan, O., Oxydation of Organic Alcohols Using a Vanadium Substituted Manganese Oxide Catalyst, Bethel College Senior Seminar, 2009, 1-51. 2. Kenneth, D. M.; Hutchison, J. E. Green Organic Chemistry: Strategies, Tools, and Laboratory Experiements, Brooks and Cole: USA, 2004, exp. 14, 197-200. Rojas 20 Acknowledgments Dr. Gary Histand Dr. Richard Zerger Dr. Dwight Krehbiel Organic Chemistry Students Family and Friends Rojas 21 Appendix 1- Organic Chemistry Class Procedure H-K-OMS-2 OXIDATION OF 9-FLUORENOL TO 9-FLUORENONE Background: In this experiment, 9-fluronone is prepared by the oxidation of 9-fluorenol. The oxidation is carried out by using an oxidative catalyst called H-K-OMS-2* (octahedral molecular sieves). Even though the toxicity of this catalyst is unknown, it is believe to be less toxic and corrosive than the typical oxidizing reagents used for this reaction, such as 𝐶𝑟𝑂3. Overall Reaction: Procedure: Set up a reflux apparatus, with a heating pad and a stirrer underneath it. In a 100 ml round bottom flask (RBF), add 0.4 g of H-K-OMS-2 and 1 mmol of the alcohol (9fluorenol). Then add 10 ml of the solvent toluene into the 100 ml RBF and analyze the reaction with thin layer chromatography (TLC) at zero time. Use 30% acetone in hexanes to elute TLC silica plates. Reflux and stir reaction at about 100℃ for 20 minutes. Check reaction for completion after the 20 minutes with TLC. If the reaction is not complete, let it reflux and stir for another 5 to 10 minutes. Once the reaction is complete cool it to room temperature and then filtrate the catalyst using gravity filtration and wash it with small amount of toluene. Save the catalyst and remove the solvent by using a rotary evaporator. Once the solvent is gone, all we have is crude product, record the crude product’s mass. Recrystallize the crude product from ethanol or ethanol/water. Record the mass of the recrystallized product and its melting point. *OMS-2 is a 2x2 octahedral, porous molecule that has square channels that are 4.6Å across.