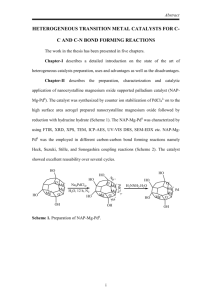
Physical Chemistry 20130419 week 3 Friday April 19 2013
advertisement

Physical Chemistry 20130419 week 3 Friday April 19, 2013 page 1 experimental rate law r= k 1 k 2 [M][A] =k uni [A] k -1 [M]+k 2 k uni = lim k uni P→∞ lim k uni P→0 at high P: at low P: k 1 k 2 [M] k1k2 = k [M]+k k -1 2 k -1 + 2 [M] k1k2 k2 since →0 [M] k -1 at high P k 1 k 2 k 1 k 2 [M] = =k 1 [M] k2 k2 [M] robs =k uni [A]= at low P k1k2 [A]=k[A] k -1 robs =k uni [A]=k 1 [M][A] 1st order 2nd order Consider a graph with kuni on the vertical axis and P on the horizontal axis. A curve comes up and right in a straight line for a while, indicating 2nd order, then its slope decreases and makes a curve, indicating 1st order. Catalyst: A substance (or species) that speeds up the rate of a chemical reaction without being consumed. Consider a graph with E(energy) on the vertical axis and reaction coordinate on the horizontal axis. A curve comes from the left side horizontally then curves up to a peak and curves back down to level out at a value lower than the starting point. The left starting point is the energy of the reactants. The right ending point is the energy of the products. The top of the curve is the energy of the uncatalyzed reaction. A second curve is exactly like the first but the peak is lower. This is for the catalyzed reaction. A third curve is like the first but has two peaks below the others. This is for a catalyzed reaction with an intermediate. A catalyst doesn’t technically change Ea. A catalyst creates its own pathway with a lower Ea. R is reactant. P is product. I is intermediate. c is catalyst. An intermediate doesn’t exist at the beginning. An intermediate is created during the reaction. A catalyst exists before the reaction starts. step 1: R1+C→I + P1 step 2: I + R2 → P2 + C total: R1 + R2 → P1 + P2 homogeneous catalysis: The catalyst and the reaction mixture are in one phase. ex) hydrolysis of an ester, everything is liquid heterogeneous catalysis: more important in industry, the catalyst and the reaction mixture form more than one phase (usually 2 phases) ex) C2 H4 +H2 Ni C H → 2 6 A catalyst can’t change the equilibrium constant. Kc= kf kr K c is constant A catalyst does change the composition (mole fractions) since it changes ∆G. ∆G°=-RTlnkp°. kp is equilibrium constant. Autocatalytic: product speeds up reaction H+ ester+ H2 O ⇀ ↽ carboxylic acid + ROH negative catalysts (inhibitors): These substances decrease the rate either by destroying the catalyst present or may react with reaction intermediates. ex) CH3∙ + ∙NO → CH3-NO 2 free radicals to nitrosomethane ∙NO has very complicated chemistry nuclear decay: first order kinetic Nuclear decay happens when the nucleus of an atom is unstable. -dN αN dt N is the number of nuclei λ is decay constant, proportionality constant -dN =λN dt then integrate: N t dN =-λ ∫ dt N0 N 0 ∫ N ln ( ) =-λt N0 N=N0e-λt A=λN radioactive materials, multiply both sides by λ A is activity A0=λN0 λN=λN0e-λt λ= .693 t 1⁄ 2 t 1⁄ is half life 2 Activity is reported in disintegrations/s = dps s is seconds A ZX X is symbol of element, Z is atomic number(number of protons),A is mass number A=Z+N N is the number of neutrons Why are some isotopes radioactive? It depends on the proton/neutron ratio. 12 6C 13 6C is stable is stable 14 6C is radioactive beta decay: 14 6C → 147N+ -10e+ ν̅ e 0 -1e ̅ , an electron or beta particle is the same as β ν̅ e is electron neutrino net ionic equation in context of nuclear chemistry: 0 0 1 1 ̅ 0n→ 1p+ -1e+ 0ν 1 0n is 12 7N 0 0 12 12 7N→ 6C+ 1e+ 0ν 0 1e neutron, 11p is proton, -10e is electron has not enough neutrons is the same as β+ , a positron or beta plus particle, 00ν is electron neutrino alpha decay: 238 234 4 92U→ 2He+ 90Th 4 2He is α(alpha)particle (nucleus only) t 1⁄ =4.5*109 years for 238 92U 2