Princeton 2012/Barron 4th ed. AP Practice Problems Unit 10
advertisement

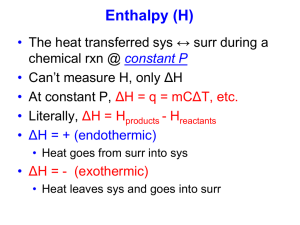
Princeton 2012/Barron 4th ed. AP Practice Problems Unit 10 – Thermochemistry Multiple Choice (no calculator) For questions 1-3, one or more of the following responses will apply; each response may be used more than once or not at all in these questions. A. B. C. D. E. 6. Which of the following is true of the reaction shown in the diagram below? (P8.6) Free energy change (∆G) Entropy change (∆S) Heat of vaporization Heat of fusion Heat capacity 1. If this has a negative value for a process, then the process occurs spontaneously. (P8.1) 2. This is a measure of how the disorder of a system is changing. (P8.2) 3. This is the energy given off when a substance condenses. (P8.3) 4. This is the energy taken in by a substance when it melts. (P8.4) 5. The reaction below is not spontaneous under standard conditions, but becomes spontaneous as the temperature decreases towards absolute zero. Which of the following is true at standard conditions? (P8.5) 2 Al(s) + 3 Cl2(g) 2 AlCl3(s) a. b. c. d. e. ∆S and ∆H are both negative ∆S and ∆H are both positive ∆S is negative, and ∆H is positive ∆S is positive, and ∆H is negative ∆S and ∆H are both equal to zero a. The reaction is endothermic because the reactants are at a higher energy level than the products. b. The reaction is endothermic because the reactants are at a lower energy level than the products. c. The reaction is exothermic because the reactants are at a higher energy level than the products. d. The reaction is exothermic because the reactants are at a lower energy level than the products. e. The reaction is endothermic because the reactants are at the same energy level as the products. Princeton 2012/Barron 4th ed. 7. Based on the information given in the table below, what is ∆H° for the above reaction? (P8.7) 10. Which point on the graph shown below corresponds to activate complex or transition state? (P8.10) 2 H2(g) + O2(g) 2 H2O(g) Bond (kJ/mol) H-H O=O O-H a. b. c. d. e. Average bond energy 500 500 500 -2,000 kJ -1,500 kJ -500 kJ +1,000 kJ +2,000 kJ 8. Which of the following is true of a reaction that becomes spontaneous at 298 K but becomes nonspontaneous at a higher temperature? (P8.8) a. ∆S° and ∆H° are both negative b. ∆S° and ∆H° are both positive c. ∆S° is negative, and ∆H° is positive d. ∆S° is positive, and ∆H° is negative e. ∆S° and ∆H° are both equal to zero 9. Which of the following will be true when a pure substance in liquid phase freezes spontaneously? (P8.9) a. ∆G, ∆H, and ∆S are all positive b. ∆G, ∆H, and ∆S are all negative c. ∆G and ∆H are negative, but ∆S is positive d. ∆G and ∆S are negative, but ∆H is positive e. ∆S and ∆H are negative, but ∆G is positive a. b. c. d. e. 1 2 3 4 5 11. C(s) + O2(g) CO2(g) ∆H° = -390 kJ/mol H2(g) + ½ O2(g) H2O (l) ∆H° = -290 kJ/mol 2 C(s) + H2(g) C2H2(g) ∆H° = +230 kJ/mol Based on the information given above, what is ∆H for the following reaction? (P8.11) C2H2(g) + 5/2 O2(g) 2 CO2(g) + H2O(l) a. b. c. d. e. -1,300 kJ -1,070 kJ -8,40 kJ -780 kJ -680 kJ Princeton 2012/Barron 4th ed. 12. If an endothermic reaction is spontaneous at 298 K, which of the following must be true for the reaction? (P8.12) I. ∆G is greater than zero II. ∆H is greater than zero III. ∆S is greater than zero a. b. c. d. e. I only II only I and II only II and III only I, II, and III 13. C(s) + 2H2(g) CH4(g) ∆H = x C(s) + O2(g) CO2(g) ∆H° = y H2(g) + ½ O2(g) H2O(l) ∆H° = z Based on the information given above, what is ∆H for the following reaction? (P8.14) 15. In which of the following reactions is entropy increasing? (P8.16) a. 2 SO2(g) + O2(g) 2 SO3(g) b. CO(g) + H2O (g) H2(g) + CO2(g) c. H2(g) + Cl2(g) 2 HCl(g) d. 2 NO2(g) 2 NO(g) + O2(g) e. 2 H2S(g) + 3 O2(g) 2 H2O(g) + 2 SO2(g) 16. When pure sodium is placed in an atmosphere of chlorine gas, the following spontaneous reaction occurs. (P8.17) 2 Na(s) + Cl2(g) 2 NaCl(s) Which of the following statements is true about the reaction? I. II. III. CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) a. b. c. d. e. x+y+z x+y-z z + y- 2x 2z + y - x 2z + y - 2x 14. For which of the following processes will ∆S be positive? (P8.15) I. NaCl(s) Na+(aq) + Cl-(aq) II. 2 H2(g) + O2(g) 2 H2O(g) III. CaCO3(s) CaO(s) + CO2(g) a. b. c. d. e. I only II only I and II only I and III only I, II, and III a. b. c. d. e. ∆S > 0 ∆H < 0 ∆G > 0 I only II only I and II only II and III only I, II, and III 17. Gaseous hydrogen and fluorine combine in the reaction above to form hydrogen fluoride with an enthalpy change of -540 kJ. What is the value of the heat of formation of HF(g)? (P8.18) H2(g) + F2(g) 2 HF(g) a. b. c. d. e. -1,080 kJ/mol -540 kJ/mol -270 kJ/mol 270 kJ/mol 540 kJ/mol Princeton 2012/Barron 4th ed. 18. Which of the following is true of the reaction shown below at room temperature? (P8.19) H2O(s) H2O(l) I. II. III. a. b. c. d. e. ∆G is greater than zero ∆H is greater than zero ∆S is greater than zero II only III only I and II only I and III only II and III only 19. 2 S(s) + 3 O2(g) 2 SO3(g) ∆H = +800 kJ/mol 2 SO3(g) 2 SO2(g) + O2(g) ∆H = -200 kJ/mol Based on the information given above, what is ∆H for the following reaction? (P8.20) S(s) + O2(g) SO2(g) a. b. c. d. e. 300 kJ 500 kJ 600 kJ 1,000 kJ 1,200 kJ Princeton 2012/Barron 4th ed. Essays 1. The reaction above proceeds spontaneously from standard conditions at 298K. (P8.4) 2 H2(g) + O2(g) 2 H2O(l) a. Predict the sign of the entropy change, ∆S°, for the reaction. Explain. b. How would the value of ∆S° for the reaction change if the product of the reaction was H2O(g)? c. What is the sign of ∆G° at 298 K? Explain. d. What is the sign of ∆H° at 298 K? Explain. 2. The reaction above is spontaneous at 298 K, and the heat of reaction, ∆H°, is -178 kJ. (P8.5a, b, c) a. Predict the sign of the entropy change, ∆S°, for the reaction. Explain. b. What is the sign of ∆G° at 298 K? Explain. c. What change, if any, occurs to the value of ∆G° as the temperature is increased from 298 K? 3. Substance C6H12O6(s) O2(g) CO2(g) H2O(l) Absolute Entropy, S (J/mol-K) 212.13 205 213.6 69.9 Molecular Weight 180 32 44 18 Energy is released when glucose is oxidized in the following reaction, which is a metabolism reaction that takes place in the body. (P8.1a, b, d) C6H12O6(s) + 6 O2(g) 6 CO2(g) + 6 H2O(l) The standard enthalpy change, ∆H°, for the reaction is -2,801 kJ at 298 K. a. Calculate the standard entropy change, ∆S°, for the oxidation of glucose. b. Calculate the standard free energy change, ∆G°, for the reaction at 298K. c. How much energy is given off by the oxidation of 1.00 gram of glucose? Princeton 2012/Barron 4th ed. 4. Bond C-H O=O C=O O-H Average Bond Dissociation Energy (kJ/mol) 415 495 799 463 CH4(g) + 2 O2(g) CO2(g) + 2 H2O (g) The standard free energy change, ∆G°, for above is -801 kJ at 298 K. (P8.1a, c, d) a. Use the table of bond dissociation energies to find ∆H° for the reaction above. b. What is the value of ∆S° for the reaction at 298 K? c. Give an explanation for the size of the entropy change found in (b). 5. The heat of formation, ∆H°f, of NH3(g) is -46.2 kJ/mol. The free energy of formation, ∆G°f, of NH3(g) is -16.7 kJ/mol. (P8.3) N2(g) + 3 H2 (g) ↔ 2 NH3(g) a. What are the values of ∆H° and ∆G° for the reaction? b. What is the value of the entropy change, ∆S°, for the reaction above at 298 K? c. As the temperature is increased, what is the effect on ∆G for the reaction? How does this affect the spontaneity of the reaction? d. At what temperature can N2, H2, and NH3 gases be maintained together in equilibrium, each with a partial pressure of 1 atm? Princeton 2012/Barron 4th ed. Answer Key – Thermochemistry Multiple Choice 1. A 2. B 3. C 4. D 5. A 6. C 7. C 8. A 9. B 10. C 11. A 12. D 13. D 14. D 15. D 16. B 17. C 18. E 19. A Essays 1. A. Negative; the products are less random than the reactants. B. ∆S° would increase because H2O(g) is more random than water, but remaining negative because the entropy would still decrease from reactants to products. C. ∆G° is negative because the reaction proceeds spontaneously. D. ∆H° must be negative at 298 K. For a reaction to occur spontaneously from standard conditions, either ∆S° must be positive or ∆H° must be negative. This reaction is spontaneous although ∆S° is negative, so ∆H° must be negative. 2. A. ∆S° is negative because the products are less random than the reactants. That’s because two moles of reactants are converted to one mole of products and gas is converted into solid in the reaction. B. ∆G° is negative because the reaction proceeds spontaneously. C. Use ∆G° = ∆H° - T∆S° ∆G° will become less negative because as temperature is increased, the entropy change of a reaction becomes more important in determining its spontaneity. The entropy change for this reaction is negative, which discourages spontaneity, so increasing temperature will make the reaction less spontaneous, thus making ∆G° less negative. 3. A. 259 J/K (Use ∆S° = ∑∆S°products - ∑∆S°reactants) B. -2,880 kJ (Use ∆G° = ∆H°-T∆S°) C. 15.6 kJ (Use stoichiometry) 4. A. -800 kJ (Use ∆H° = ∑Energies of bonds broken - ∑Energies of bonds formed) B. 3 J/K (Use ∆G° = ∆H°-T∆S°) Princeton 2012/Barron 4th ed. C. ∆S° is very small, which means that the entropy change for the process is very small. This makes sense because the number of moles remains constant, the number of moles of gas remains constant, and the complexity of the molecules remains about the same. 5. A. -33.4 kJ (Use ∆G° = ∆H°-T∆S°) B. -198 J/K (Use ∆G° = ∆H°-T∆S°) C. From (B), ∆S° is negative, so increasing the temperature increases the value of ∆G°, making the reaction less spontaneous. D. 467 K (Use ∆G° = ∆H°-T∆S°)