Equilibrium worksheet answers - SCH4U1-CCVI
advertisement

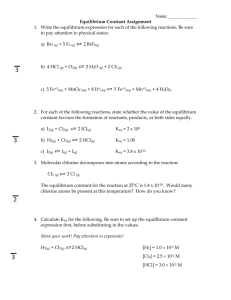
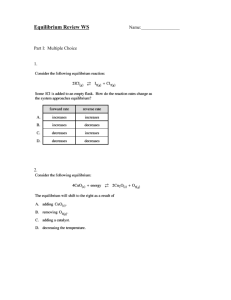
Equilibrium Constant, Keq 1. Once a system has reached equilibrium, are the following true or false? a. The reaction is finished, no more products are forming. ____false______ b. The concentrations of the reactants and the products are equal. ____false_______ c. The concentrations are no longer changing. ____false__ d. The reaction is not over, but will continue forever if isolated. ____true____ e. The speed at which products are made equals the speed at which reactants form. ___true___ 2. What is equal at equilibrium? _______rate forward = rate reverse______________ 3. What general information can be gathered by observing the magnitude of the equilibrium constant? Whether the reactants or products are favoured. 4. Write the expression for Keq for the reaction: 2 NO (g) + Cl2 (g) 2 NOCl (g) 2 K eq [NOCl] [NO]2 [Cl2 ] 5. Write the Keq for: 2 K3PO4 (aq) + 3 Ca(NO3)2 (aq) 6 KNO3 (aq) + Ca3(PO4)2 (s) K eq [KNO3 ]6 [K 3PO 4 ]2 [Ca(NO3 ) 2 ]3 6. Write the expression for Keq for the reaction : K eq H2 (g) + Br2 (l) 2 HBr (g) [HBr] 2 [H 2 ] 7. Write the expression for Keq for the reaction: K eq CO2 (g) + CaO (s) 1 [CO 2 ] 8. For the reaction: SiH4 (g) + 2 O2 (g) SiO2 (g) + 2 H2O (l) a) Write the equilibrium expression for the forward reaction: K eq [SiO 2 ] [SiH 4 ][O2 ]2 b) Write the equilibrium expression for the reverse reaction: K eq ' [SiH 4 ][O 2 ]2 1 [SiO 2 ] K eq CaCO3 (s) c) What is the equilibrium constant in the forward direction if [SiH4] = 0.45M; [O2] = 0.25M; and [SiO2] = 0.15M at equilibrium? K eq [SiO 2 ] [SiH 4 ][O2 ]2 0.15 (0.45)(0.25) 2 5.3 d) What is the equilibrium constant in the reverse reaction? 1 0.19 5.3 K eq ' e) If [SiH4] = 0.34M; [O2] = 0.22M and [SiO2] = 0.35M, what would be the reaction quotient (Q) in the forward direction and which direction will the reaction go? K eq 0.35 21 (0.34)(0.22) 2 Q = 21 > Keq = 0.053 then the reaction will go towards the reactants Calculating Keq, Q, ICE tables 9. Calculate the equilibrium constant for this reaction: 2 PO2Br (aq) 2 PO2 (aq) + Br2 (aq) Given: [PO2Br] = 0.0255M, [PO2] = 0.155M, and [Br2] = 0.00351M at equilibrium. 2 PO 2 Br 2 PO 2 Br2 K eq (0.155) 2 (0.00351) (0.0255) 2 K eq [PO 2 ]2 [Br2 ] [PO 2 Br] 2 0.130 10. For the combination reaction: H2 (g) + F2 (g) 2 HF (g), calculate all three equilibrium concentrations when [H2]i= [F2]i = 0.200 M and Keq = 64.0. H2 I 0.200 C -x E 0.200 - x K eq F2 2 HF 0.200 0 -x 2x 0.200 - x 2x K eq [HF] 2 64.0 [H 2 ][F2 ] Q 0 Keq 100 Rule - doesn' t apply 0.200 0.003125 100 64.0 (2x) 2 64.0 perfect square (0.200 - x)(0.200 - x) (2x) 2 64.0 (0.200 - x)(0.200 - x) [H 2 ]eq 2x 8.00 0.200 - x 2x 1.60 - 8.00x 10.00x 1.60 1.60 x 0.160 10.00 [F2 ]eq 0.200 - 0.160 0.040 M and [HF]eq 2(0.160) 0.320 M (0.320) 2 Check : Q (0.040) 2 64.0 11. For the decomposition reaction, COCl2 (g) CO (g) + Cl2 (g), calculate all three equilibrium concentrations when Keq = 0.680 with [CO]o = 0.500 and [Cl2]o = 1.00 M. CO Cl2 I 0 C x 0.500 -x 1.00 -x E x 0.500 - x COCl2 K eq K eq [CO][Cl2 ] 0.680 [COCl2 ] Q undef Keq 100 Rule - doesn' t apply 0.500 1.00 - x 0.735 100 0.680 (0.500 - x)(1.00 - x) 0.680 x 0.500 - 1.50x x 2 0.680 x x 2 - 2.18x 0.500 0 a 1 b - 2.18 c 0.500 - (-2.18) 4.7524 - 2 2 2.18 1.66 x 2 x 1.92 (too big) or x 0.260 x [COCl2 ]eq 0.260M , [CO]eq 0.500 - 0.260 0.240 M and [Cl 2 ]eq 1.00 - 0.260 0.740 M Check : Q (0.240)(0. 740) 0.683 (0.260) 12. We place 0.0640 mol N2O4 (g) in a 4.00 L flask at 200 K. After reaching equilibrium, the concentration of NO2(g) is 0.00300 M. What is Keq for the reaction N2O4(g) 2 NO2(g)? [NO 2 ]2 N 2O 4 2 NO2 K eq [N 2O 4 ] I 0.0160 0 C -x 2x E 0.0160 2x but [NO 2 ]eq 0.00300 M 2x 0.00300 x 0.00150 (0.00300) 2 6.21 x 10-4 (0.0160 - 0.00150) K eq 13. Carbonyl bromide decomposes to carbon monoxide and bromine: COBr2(g) CO(g) + Br2(g) Keq is 1.90 x 10-4 at 73oC. If an initial concentration of 0.330 M COBr2 is allowed to equilibrate, what are the equilibrium concentrations of COBr2, CO, and Br2? COBr2 CO Br2 0 0 I 0.300 C -x x x E 0.300 - x x x K eq [CO][Br2 ] 1.90 x 10-4 [COBr2 ] Q 0 Keq 100 Rule - applies 0.300 1579 100 1.90 x 10-4 0.300 - x 0.300 K eq ( x)( x) 1.90 x 10-4 0.300 x 2 5.70 x 10-5 [COBr2 ]eq x 7.55 x 10 -3 0.300 - 0.00755 0.292 M , [CO]eq [Cl 2 ]eq 0.00755 M (0.00755) 2 Check : Q 1.93 x 10-4 0.292 14. PCl5 decomposes into PCl3 and Cl2 gas. What is the initial concentration of PCl5 if at equilibrium the concentration of chlorine gas is 0.500M? Given: Keq = 10.00 (Hint: ICE table) PCl5 I y C -x PCl3 Cl2 0 0 x x E y-x x and [Cl 2 ]eq 0.500M x K eq K eq [PCl3 ][Cl 2 ] 10.00 [PCl5 ] x (0.500)(0. 500) 10.00 y - 0.500 0.25 10.00y - 5.00 10.00y 5.25 y 0.525 [PCl5 ]i 0.525 M and [PCl5 ]eq 0.525 - 0.500 0.025 M Check : Q (0.500)(0. 500) 10.00 0.025 Le Chatelier’s Principle 15. Consider this endothermic reaction: 3 O2(g) 2 O3(g). To shift this reaction to the reactants: a) b) c) d) e) You could ________ the pressure. (decrease) You could ________ the volume. (increase) You could ________ oxygen gas. (remove) You could ________ the temperature. ( increase or decrease) Which of the following, if increasing, will change the value of the equilibrium constant? d) Temperature 16. Consider this reaction: 2 SO2(g) + O2(g) 2 SO3(g). To shift this reaction towards the products: a) You could _________ the pressure. (increase) b) You could _________ the volume. (decrease) c) You could _________ oxygen gas. (add) 17. For each system described below, indicate in which direction the equilibrium will shift when each stress is added or removed. a) N2 (g) + 3 H2 (g) 2 NH3 (g): more H2 is added to reaction at equilibrium. () b) For the same reaction, some NH3 is removed from the reaction at equilibrium. () c) 2 SO2 (g) + O2 (g) 2 SO3 (g) + heat: heat is added. () d) Using the same reaction, heat is removed () e) PCl3 (g) + Cl2 (g) PCl5 (g): volume is reduced by half. () f) Using the same system as above, a catalyst is added to the system. (same) g) H2 (g) + Cl2 (g) 2 HCl (g): volume is doubled. (same) h) Using the same system as above, some neon is added to the system. (same) 18. Explain how the following changes in reaction conditions will affect the position of the equilibrium: A (g) + B (aq) C (s) ΔHrxn= -453 kJ/mol a) The pressure of A in the reaction chamber is increased. () b) The temperature of the reaction is increased by 20 0C. () c) A catalyst is added to the system. (same) d) More of compound B is steadily added to the reaction chamber. () e) More of compound C is steadily added to the reaction chamber. (same) f) Argon gas is added to the reaction chamber, doubling the pressure. ()