Skeletal Muscle
advertisement

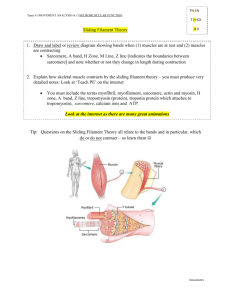
Muscle Skeletal muscle Skeletal muscle comprises the largest group of tissues in the animal body and accounts for up to 40% of total body weight. This type of muscle, which is innervated by the somatic nervous system, is under voluntary control. Skeletal muscle performs many important functions in the body, including: • Movement of body parts • Heat production • Breathing • Speaking Structure of skeletal muscle A whole muscle is composed of muscle cells, or muscle fibers. Muscle fibers are elongated, cylindrical cells. Due to fusion of many smaller fibers during embryonic development, muscle fibers are the largest cells in the body, with several nuclei near their surface. Muscle fibers lie parallel to each other and extend along the entire length of the muscle. These fibers may be a few millimeters in length (muscles of the eyes) or up to 2 or more feet in length (muscles of the legs). Muscle fibers are incapable of mitosis. Because muscle fibers have no gap junctions between them, electrical activity cannot spread from one cell to the next. Therefore, each muscle fiber is innervated by a branch of motor neuron. A motor unit is defined as a motor neuron and all of the muscle fibers that it innervates. Internally, muscle fibers are highly organized. Each fiber contains numerous myofibrils — cylindrical structures that also lie parallel to the long axis of the muscle. The myofibrils are composed of thick filaments and thin filaments. It is the arrangement of these filaments that creates alternating light and dark bands observed microscopically along the muscle fiber. Thus, skeletal muscle is also referred to as striated muscle. Sarcomeres .The thick and thin filaments are organized into repeating segments referred to as sarcomeres , which are the functional units of skeletal muscle. (In other words, the sarcomere is the smallest contractile unit within skeletal muscle.) A myofibril is composed of hundreds or thousands of sarcomeres in series along its length. When a muscle is stimulated, each of these sarcomeres contracts and becomes shorter. As a result, the entire muscle contracts and becomes shorter. Therefore, the function of the sarcomere determines whole muscle function. A sarcomere is the area between two Z lines. The function of the Z line is to anchor the thin filaments in place at either end of the sarcomere. The thick filaments are found in the central region of the sarcomere. Ultimately, interaction between the thick and thin filaments causes shortening of the sarcomere. Thick filaments. Each thick filament contains 200 to 300 myosin molecules. Each myosin molecule is made up of two identical subunits shaped like golf clubs: two long shafts wound together with a myosin head, or crossbridge, on the end of each. These molecules are arranged so that the shafts are bundled together and oriented toward the center of the thick filament. The myosin heads project outward from either end of the thick filament. Thin filaments. The thin filaments are composed of three proteins: • Actin • Tropomyosin • Troponin The predominant protein, actin, consists of spherical subunits (globular actin) arranged into two chains twisted around each other (fibrous actin). Tropomyosin is a long, thread-like protein found on the outer surface of the actin chain. Each tropomyosin molecule is associated with six to seven actin subunits. The function of tropomyosin is to cover binding sites for myosin on the actin subunits when the muscle is in the resting state. This prevents the interaction between actin and myosin that causes muscle contraction. Troponin is a smaller protein consisting of three subunits. One subunit binds to actin, another binds to tropomyosin, and the third binds with calcium. When the muscle is relaxed, troponin holds the tropomyosin in its blocking position on the surface of the actin . Mechanism of contraction The action potential is readily propagated, or transmitted, along the surface of the muscle fiber. However, a mechanism is needed to transmit the electrical impulse into the central region of the muscle fiber as well. The transverse tubules ( T tubules ) are invaginations of the cell membrane penetrating deep into the muscle fiber and surrounding each myofibril. As the action potential travels along the surface of the fiber, it is also transmitted into the T tubules. As a result, all regions of the muscle fiber are stimulated by the action potential. All types of muscle require calcium for contraction. In skeletal muscle, Ca++ ions are stored within an extensive membranous network referred to as the sarcoplasmic reticulum. This network is found throughout the muscle fiber and surrounds each myofibril. Furthermore, segments of the sarcoplasmic reticulum lie adjacent to each T tubule that, with a segment of sarcoplasmic reticulum on either side of it, is referred to as a triad. As the action potential is transmitted along the T tubule, it stimulates the release of Ca++ ions from the sarcoplasmic reticulum. The only source of calcium for skeletal muscle contraction is the sarcoplasmic reticulum. The mechanism of skeletal muscle contraction is described by the Sliding Filament Theory. This mechanism begins with the “ priming ” of the myosin crossbridge, a process that requires energy, which is supplied by adenosine triphosphate (ATP). Each myosin crossbridge contains myosin ATPase. When ATP attaches to its binding site on the myosin crossbridge, it is split by the myosin ATPase to yield adenosine diphosphate (ADP) and inorganic phosphate (Pi). The ADP and Pi remain tightly bound to the myosin crossbridge. Energy released by this process causes the myosin crossbridge to swivel outward toward the end of the thick filament. When the crossbridge is in this conformation, it is “primed” and referred to as the high-energy form of myosin; this form of myosin is capable of binding to actin. However, this interaction is prevented by tropomyosin, which physically covers the binding sites for myosin on the actin subunits. In order to uncover these binding sites, calcium is needed. In a stimulated muscle fiber, Ca++ ions are released from the sarcoplasmic reticulum and bind to troponin. As a result, the troponin–actin linkage is weakened, allowing the tropomyosin to be repositioned such that the myosin-binding sites are uncovered. The myosin crossbridge now binds to the actin, causing the energy previously stored within the myosin to be discharged and the crossbridge to swivel inward toward the center of the thick filament. This process is referred to as crossbridge cycling. As the myosin crossbridge swivels inward, it pulls the actin inward as well. It is important to note that the interaction between actin and myosin causes the thin filaments to slide inward over the thick filaments toward the center of the sarcomere. Consequently, the sarcomeres shorten and the whole muscle shortens or contracts. It is for this reason that this process is referred to as the Sliding Filament Theory of muscle contraction. When the myosin crossbridge binds with the actin, ADP and Pi are released from the myosin. This opens the binding site to another molecule of ATP. In fact, the myosin remains attached to the actin until another ATP molecule binds to the myosin. Binding of a new ATP causes the myosin to release the actin. This ATP is split by the ATPase and the myosin crossbridge swivels outward once again, returning the myosin to its high-energy state. As long as Ca++ ions are present and the binding sites on the actin are uncovered, crossbridge cycling continues. The crossbridges of the thick filament pull the thin filaments inward incrementally so that the sarcomeres become even shorter and the muscle contracts further. Interestingly, the myosin crossbridges do not all cycle at the same time. At any given moment, some crossbridges remain attached to the actin and others are in the process of releasing the actin in order to cycle once again. In other words, myosin crossbridge cycling is staggered. This process maintains the shortening of the sarcomere and prevents thin filaments from slipping back to their original positions in between cycles. In the absence of ATP, myosin crossbridges are unable to release the actin. As a result, the sarcomeres, and therefore the muscle, remain contracted. This phenomenon is referred to as rigor mortis. Following death, the concentration of intracellular calcium increases. This calcium allows the contractile process between the previously formed high-energy myosin and the actin to take place. However, the muscle stores of ATP are rapidly depleted, the myosin remains attached to the actin, and stiffness ensues. When the action potentials in the alpha motor neuron cease, stimulation of muscle fiber is ended. Ca++ ions are pumped back into the sarcoplasmic reticulum and troponin and tropomyosin return to their original positions. As a result, the myosin-binding sites on the actin are covered once again. The thin filaments return passively to their original positions, resulting in muscle relaxation.