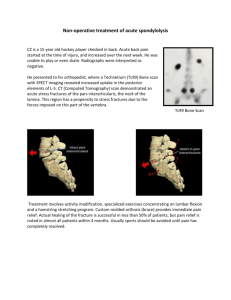
pediatric infections
advertisement

Manuscript copyright USENKO Denis Valerievych ACUTE ENTERIC INFECTIONS IN CHILDREN WITH ATOPIC DERMATITIS: CLINICAL AND IMMUNOLOGICAL FEATURES, MANAGEMENT AND REHABILITATION 14.01.09 - Infectious Diseases Thesis abstract for a doctor’s degree Doctor of Medicine Moscow-2013 The work has been performed in the Federal Budget Scientific Institution "Central Research and Development Institute of Epidemiology "of Federal service on customers’ rights protection and human well-being surveillance. Scientific adviser MD, Professor Alexander Vasylievich Gorelov Official opponents Feklisova, Lyudmila Vladimirovna- MD, professor State-financed health institution of Moscow region "Moscow Regional Research and Development Clinical Institute named after M.F. Vladimirsky", head of the department of pediatric infections Bokovoy, Alexander Grigoryevich- MD, professor Federal state budgetary educational institution of higher professional education "Moscow State University named after M.V. Lomonosov", Fundamental Medicine faculty, Professor, Department of multidisciplinary clinical training; Federal State Budgetary Institution "Central Clinical Hospital and Polyclinic» Administrative Department of the President of the Russian Federation, Head of the department of pediatric infectious Kalyuzhin, Oleg Vitalyevich - MD, professor Federal state budgetary educational institution of higher professional education « Moscow First State Medical University named after I.M. Sechenov” of Ministry of Health of the Russian Federation, Professor, Department of Clinical Immunology and allergy Leading organization: Federal state budgetary educational institution of higher professional education « Peoples’ Friendship University of Russia" Ministry of Education of the Russian Federation. Thesis defense viva voce will be held on “____” _________2013, ___ h. ____ min. in a panel session of Dissertation Council D 208.114.01 in the Federal Budget Institution of Science "Central Research and Development Epidemiology Institute " of Federal service on customers’ rights protection and human well-being surveillance situated at the address: Novogireevskaya Street 3a, 111 123 Moscow,. Those who wish can acquaint themselves with the thesis in the library of Federal Budget Institution of Science "Central Research and Development Epidemiology Institute " of Federal service on customers’ rights protection and human well-being surveillance. Author's thesis has been distributed on "____" ___________ 2013 Scientific Secretary of the Dissertation Council MD, Professor Alexander Gorelov General work characteristics Rationale. Acute intestinal infections (AII) remain the most common infectious diseases. According to World Health Organization (WHO), every year 2.5 billion cases of diarrheal disease is detected in the world, up to 70% of the patients are children aged up to 14 years [WHO, 2008]. In 2008, AII caused 15% of all deaths of children in the first five years of life on the planet, yielding the first place only to acute pneumonia (18%) [WHO, 2011]. In the Russian Federation over the past few decades, a stable tendency of increase of AII incidence rate was observed, with the mean annual rate of increase 67% [Onishchenko G.G., 2008; Mindlina A. Ya., 2010]. According to Federal service on customers’ rights protection and human well-being surveillance, in 2011 782,000 AII cases were registered, both of established and unknown etiology, of which 79.6% of age structure cases were children under 17 years of age. High frequency of severe and adverse AII course in children of different age groups as well as long-term bacterioexretion and virus shedding still remains an urgent problem of practical health care [Feklisova L.A., 2005; Mikhailova E.V. et al., 2009; Gorelov, B.C. et al., 2010; Fedotov N.N., 2009; Anokhin V.A. et al., 2012; Grigorovich M.S., 2012; Lobzin Yu.V. et al., 2012]. The reasons of this problem include violation of nutritional status, constitution anomalies, acute and chronic foci of infection (adenoiditis, tonsillitis, bronchitis, SARS), anemia, organic lesions of the central nervous system, microbiocenosis violation, minor forms of immunodeficiency diseases, functional disorders of the gastrointestinal tract (GIT) and allergic diseases. The role of allergic diseases in children has increased significantly over the recent decades [ICCAD II, 2003; Holt P.G. et al., 2010; Balabolkin I.I., 2012]. According to the results of epidemiological studies carried out abroad, the most common allergic disease in children is atopic dermatitis (AD), the frequency of which varies from 15 to 30% [BieberT, 2008]. In the Russian Federation, AD frequency in different regions varies from 10 to 37% and in infants – up to 38% [Khaitov R.M., 2001; Kondyurin E.G. et al., 2004; Revyakina V.A. et al., 2006]. Atopic dermatitis is a multifactorial disease with a basically a genetic predisposition, complicated immunopathological development mechanism, with violations both in innate and in adaptive immunity [Suvorov K.N., 1998; Fedenko E.S., 2005; Khaitov R.M., 2009; Kochergin N.G. et al., 2010; Mar A., 2010] In children with AD, neuroendocrine abnormalities are detected as well as metabolic disturbance and digestive organ dysfunction [Illek Ya.Yu. et al. 2003; Shutova O.V., 2006; Balabolkin I.I., 2006; Galanin A.V., 2008], which maintains chronic disease. The problem of simultaneous course of two common pathological states is acute, because immune disbalance and damage of GIT microbiocenosis resulting from this combination may lead to a change of clinical symptoms, and the low efficiency of conventional treatment [Kvetnoy AS et al., 2008]. So far, immunological aspects of AII development, with concurrent allergic diseases still have to be examined, besides presence of immunity disorders inherent to the allergic process and variations of immune homeostasis caused by bacterial and viral agents should be considered as well [Martynov G.P., 2003, Maikova I.D., 2008]. Mechanisms of cytokine regulation under the influence of infectious antigens have not been fully studied. Levels of the main pro- and anti-inflammatory cytokines were actively studied in children with AII [Beniova S.N. et al. 2007; Gussoyeva I.G., 2008; Zheleznikova G.F., 2009], whereas the effect of background allergic diseases on production of key immune mediators are almost unexplored. Among the questions that remain unexplored are Influence of morphological and functional as well as microecological GIT disorders in patients with allergic diseases on AII course as well as possibility of manifestation of allergic lesions of the digestive tract simulating acute infectious gastroenteritis / gastroenterocolitis" To date there are no coordinated approaches to the treatment of this category of patients in the medical practice. Attempts have been made to implement probiotics and enterosorbents in complex treatment, but there are only few works in this area. Tactics of antihistamine AII therapy in children with atopic dermatitis requires further development. Rehabilitation of children with AD undergoing AII requires separate consideration. Given the high frequency of postinfectious bowel dysfunction and microflora disorders, use of biocenosis-correcting technologies, in particular modern probiotic foods is a promising approach to the timely correction of such damages, improvement of the quality of life and favorable disease outcomes [Burkin A.V., 2005; Elezova L.I., 2005; Loverdo R.G. et al., 2009]. Objectives: to optimize diagnosis and treatment of acute intestinal infections in children with atopic dermatitis based on identification of clinical and pathogenic characteristics and study of the state of intestinal microbiocenosis. Research tasks: 1. To determine the frequency of concomitant allergic diseases in children hospitalized with acute intestinal infection. 2. To study clinical and laboratory features of acute intestinal infections in children with atopic dermatitis, to evaluate effect of various factors (age, etiopathogenic variant of intestinal infection, type and severity of atopic dermatitis). 3. To investigate performance of cellular, humoral and mucosal immunity, cytokine status in children with acute intestinal infection suffering from atopic dermatitis according to etiology and severity infectious process, genesis and severity of allergic disease. 4. To examine intestinal microbiocenosis status in AAI children with AD considering their age and disease severity. 5. To assess clinical and laboratory efficiency of modern enterosorbents, probiotics and antihistamines in AAI children suffering from atopic dermatitis, and to justify the differential use of these drugs for treatment and rehabilitation. Scientific novelty: New data have been received on the role of allergic diseases in the structure of concomitant somatic pathology of children with acute intestinal infections. A possibility appeared of manifestation of allergic GIT disease simulating AII, and tactics of differential diagnostic search have been proposed. The first studies have been conducted for AD effect on the clinical features, course and outcomes of AII in children. A high frequency of wave-like and prolonged AII course has been demonstrated, as well as prevalence of moderate to severe forms, high symptom load (intoxication exsicosis, diarrheal syndrome) and duration of clinical manifestations, persistent gastrointestinal disorders, secondary disaccharidase deficiency and dysbiosis in the convalescence period. New data have been received on the state of cellular and humoral immunity, phagocytic system, gastrointestinal mucosal immune system in children in various periods of acute intestinal infection with various immunopathological AD variants. It has been detected that patterns of AII against the background of atopic dermatitis are characterized by violations of immunoregulatory mechanisms whose severity and direction depends on the type of pathogen and severity of the disease. It has been established that immunopathogenic factors causing unfavorable clinical course in AD children with acute intestinal infections is disbalance of quantitative parameters of cellular immunity, with increase in the ratio of CD4 + - and CD8 + - cells; deficiency of serum IFN-γ; high IgE serum levels, reduction of factors of local GIT protection, particularly free and secretory IgA. For the first time differences have been revealed in the patterns of cytokine regulation immune response in AII on the background of atopy and without concomitant allergic pathology. It has been shown that in AII of viral etiology, Th2-type of immune response dominates in patients with atopic dermatitis, manifested in significant increase in the levels of IL-4, with parallel IFN-γ deficiency, whereas in patients without allergy pathologies a balanced Th1 / Th2-immune response is determined. Based on the first evaluation of allergy indicators (total IgE and specific IgE antibodies to opportunistic microorganisms) in AII dynamics, the data have been received showing their multidirectional character: sensitization growth after undergoing AII of viral etiology and a downward trend of IgE level in convalescence from bacterial AII. For the first time negative impact of AD has been shown on the depth and durability of microecological disorders in the GIT in pediatric intestinal infections. It has been established that their expression is directly correlated with the severity of atopic dermatitis. Note that frequency of self-relief of GIT microbiocenosis disorders arising from AII in children with atopy has not suceeded 5,2%. For the first time, pathogenic substantiation has been given, and comparative assessment of efficiency of modern enterosorbents, probiotics and antihistamines in AII has been conducted in children with atopic dermatitis. Therapy and rehabilitation tactics of these patients have been developed. Practical significance: • Clinical and laboratory features of acute intestinal infections have been identified in children with concomitant AD, allowing clinicians to predict the course of infection and adjust the therapy in time. • A set of clinical and laboratory parameters has been proposed for differential diagnosis of acute intestinal infections and manifestation of gastrointestinal food allergy in infants. • These features of the immune response in AII of various etiology on the background of AD increase the possibility of assessing severity of the course and prognosis of the disease. For this purposes, monitoring of proinflammatory (IFN-γ, IL-1β) and anti-inflammatory (IL-4) cytokines, leukocyte index (IFN-γ / IL-4), serum levels of total IgE, levels of secretory IgA and free IgE in the coprofiltrate is recommended. • Based on the new data on efficiency of modern enterosorbents, probiotics and antihistamines, recommendations have been developed to optimize their use for the treatment of AII children with atopic dermatitis, which can improve the prognosis and outcome of acute intestinal infections in this category of patients. • A complex of rehabilitation measures, including use of antihistamines of II generation, probiotics and products of functional foods has been proposed to correct postinfectious functional GIT disorders and effects of dysbiosis, to reduce sensitization and AD progression. Evaluation of the research results Results of the study have been presented at the IX Congress of Pediatricians of Russia "Actual problems of Pediatrics" (2004), Russian national scientific and practical conference "Treatment of infectious diseases in children: modern conceptions and unsolved problems "(2005), Russian national conference "Topical issues of Pediatrics" (2005); VII-IX Congress of Russian pediatric infectious diseases (2008-2010); XIV Russian Gastroenterological Week (2008), I-IV Annual Russian Congress on Infectious Diseases (2009-2012), XII -XIV Russian Congress of Dietitians and Nutritionists "Nutrition and Health" (2010-2012), X and XI Russian Congress of Pediatricians specializing in infectious disease (2011, 2012), III Interregional Scientific and Practical Conference "Infectious diseases: topical issues of diagnosis, treatment and prevention "(2012). The dissertation has been approved in a panel session of Dissertation Council D 208.114.01 in the Federal Budget Institution of Science "Central Research and Development Epidemiology Institute " of Federal service on customers’ rights protection and human well-being surveillance on November 26, 2012. Publications The main scientific results on the dissertation topic have been published in 47 printed works, of them 24 are journals recommended by State Commission for Academic Degrees and Titles for publication of major scientific results of dissertations. Implementation of the research results The results have been applied in the direct of specialized departments of National public institution of health care Hospital for the infectious diseases of children №5, Moscow Department of health, as well as included in the "Practice guidelines to diagnosis and comprehensive treatment of acute intestinal infections in children "(Study guide for physicians. Moscow, 2003), "Clinical practice for diagnosis and treatment of acute intestinal infections in children "(Study guide for physicians. Ministry of Health and Social Development of the Russian Federation. Moscow, 2005), "Practice Guidelines for antibiotic shigellosis therapy in children" (Study guide for physicians. Moscow, 2006). Thesis structure The work is presented on 331 pages of typescript text and consists of introduction, four chapters and reference list. The work contains 47 figures and 75 tables. The source book contains 470 reference links, including 197 of Russian and 253 of foreign authors. CONTENT OF THESIS Materials and Methods The thesis was written in the clinical department of infectious pathology Federal Budget Institution of Science "Central Research and Development Epidemiology Institute" of Federal service on customers’ rights protection and human well-being surveillance in the years 2002-2010. To achieve the goals and solve the tasks, clinical and laboratory observations of 476 patients (boys - 60.4%, girls 39.6%) at the age from 3 months to 14 years hospitalized in the specialized departments of National public institution of health care Hospital for the infectious diseases of children №5, Moscow Department of health (Chief executive officer Vlasov EV.). have been performed. The main group consisted of 231 children who had previously been diagnosed with atopic dermatitis (according to the form 112 / u "Infant record", approved by the Order № 1030of USSR Ministry of Health from October 4, 1980). 245 patients who did not have any data on concomitant allergic pathology in the history and at the time of admission were chosen by random sampling technique for the comparison group of 3409 children patients treated in the hospital during the period 2002-2010. Among the patients surveyed, 33.3% were children of the first year of life, 26% - aged from 1 year to 3 years, 40.7% - older than 3 years. All the patients were diagnosed with acute intestinal infection based on history, clinical and epidemiological data, results of bacteriological, serological, molecular genetic studies considering the diagnostic criteria adopted in the hospital. Mild AII course was observed in 21% of children, moderate to severe - in 60.4%, severe – in 18.6%. In 77.3% cases the patients were hospitalized within the first three days of illness (day 1 - 31.4%, day 2 - 27.3%, day 3 – 18.6%). According to the disease topography of GIT, in the patients observed gastroenteritis form prevailed (41.1%), more seldom the pathological process involved distal segments of the intestine, with the development of enterocolitis (19.9%) and gastroenterocolitis (22.5% patients). Among the surveyed patients of the main group, mild AD course was observed in 48.5% (112 children) - 1-2 exacerbations per year, remission duration 6-8 months. Moderate course of the disease (exacerbation frequency 3-4 times a year, remission duration 2-3 months) was established in 44.2% (102 patients), and severe course - in 7.3% (17 children) (not less than 5 exacerbations in the past year, with brief remission in 1-2 months or persistent disease). In assessing AD severity, criteria of scientific and practical program "Atopic dermatitis and skin infections in children: diagnosis, treatment and prevention "(Union of pediatricians of Russia, 2004) were used as a guide. Etiology of acute intestinal infections defined using a set of diagnostic methods (bacteriological, serological, molecular-genetic), was determined in 75.6% children. rotaviruses were the leading cause of the disease - 28.6%, other diarrheagenic viruses (Norwalk, astro-, adenoviruses) - 17.5%, and Salmonella - 12.6%. Much rarer causes of AII included Shigella (5.2%), Escherichia (5.2%), opportunistic pathogenic microorganisms (5%) and Campylobacter (1.5%). In every fourth patient, disease etiology was not deciphered. All patients received combined therapy according to present clinical recommendations including diet therapy, oral rehydration or infusion therapy for the main indication as well as symptomatic therapy. In accordance with the purpose and objectives of the present study, clinical and laboratory evaluation of the efficiency of modern enterosorbents in AII was conducted in 99 children with concomitant AD. Group 1 consisted of 53 patients receiving dioctahedral smectite (Smecta) from the first days of the disease; group 2 consisted of 46 patients receiving polymethylsiloxane polyhydrate (Enterosgel); group 3 (comparison group) included 26 children treated without using enterosorbents. The preparations were prescribed based on standard dosages set forth in the instructions for use. Clinical and laboratory evaluation of the effectiveness of modern probiotics in the complex therapy of acute intestinal infections was conducted in 92 children with atopic dermatitis (main group). Patients of the study group received monocomponent (Enterol - 15 patients, Probifor - 23 patients) or multicomponent (Acipol - 34 children, Linex - 20 patients) probiotics. Simultaneously, observation of 63 patients receiving no probiotics (comparison group) was conducted. The dynamics of the relief of AAI symptoms, severity of manifestations and AD course as well as intestinal microbiotic values were assessed. According to the task of identifying therapy and rehabilitation tactics in AAI children suffering from AD, effectiveness of clinical and laboratory values of antihistamines (AHP) of the first (suprastin, tavegil) and second generation (zyrtec) was assessed in complex AII therapy in children suffering from atopic dermatitis (study group). The comparison group included 59 patients receiving AHP treatment at prehospital and hospital stages. Symptom load and duration of the main symptoms of intestinal infection as well as value dynamics of activity of cutaneous allergic process and frequency of AD exacerbation was identified as effectiveness criteria. In order to improve the rehabilitation program for AII convalescents suffering from atopic dermatitis, efficiency was assessed for a set of measures including II generation AHP (course of up to 3 months.) Polycomponent probiotic (Linex) or probiotic products for functional nutrition containing Lactobacillus casei DN-114001 or Bifidobacterium animalis DN-173010 (adjusted for age, character of gastrointestinal disorders and the absence of allergy to cow milk protein). The efficiency of the proposed complex has been studied in 72 children with atopic dermatitis. Laboratory examination A standard laboratory examination included: complete blood count and clinical urinalysis, coprological examination, biochemical blood assay, determination of blood acid-base composition, feces analysis and scraping for helminth eggs and protozoa in the clinical laboratory of National public institution of health care Hospital for the infectious diseases of children №5, Moscow Department of health (Chief of laboratory Schastnych L.A.). Determination of the level of free carbohydrates in the feces was conducted in the clinical diagnostic Center of Federal Budget Institution of Science "Central Research and Development Epidemiology Institute" of Research Center of Epidemiology and Microbiology named after G.N. Gabrichevsky, Federal service on customers’ rights protection and human well-being surveillance. In order to establish AII etiology, the following procedures were conducted: single-step / threefaeces inoculation to determine pathogenic microorganisms, in case of indication - inoculation of gastric lavage and faeces on the opportunistic flora (Chief of bacteriological laboratory of National public institution of health care Hospital for the infectious diseases of children №5, Moscow Department of health Gulid E.P.), detection of rotavirus antigen in the faeces by ELISA using Rota-analysis test system of JSC "Bioimmunogen" (Moscow) (specialist - virologist Kosorotikova A.I.). On the first day of admission (but not later than 3 days from the disease onset), initial testing of faeces of patients on the presence of intestinal infection agents (Shigella and EIEC, Salmonella, Campylobacter, group A rotaviruses, noroviruses of genotype 2, astro- and adenoviruses) was performed by polymerase chain reaction using diagnostic test systems with electrophoretic amplification product detection of "AmpliSens" family (performed in the Laboratory of Molecular diagnosis and epidemiology of intestinal infections, National public institution of health care " Central Research and Development Epidemiology Institute of Federal service on customers’ rights protection and human well-being surveillance ", Chief of the laboratory, PhD Podkolzin A.T). In the second week of disease (according to indications), blood serological test was carried out by passive hemagglutination test (PHA) method with Salmonella, Shigella and Yersinia antigens (Chief of bacteriological laboratory of National public institution of health care Hospital for the infectious diseases of children №5, Moscow Department of health Gulid E.P.) Intestinal microbiocenosis investigation was conducted in the acute period (days 1-3 of the disease) and in the recovery period (days 14-16 of the disease) by method of R.V. Epstein-Litvak and F.L. Vilshanskaya in the National public institution of health care CNIIEP of Federal service on customers’ rights protection and human well-being surveillance (Chief of laboratory Bochkov I.A, MD) and FSBI "Research and Development Nutrition Institute", RAMS (Chief of laboratory Sheveleva S.A., MD). Evaluation of GIT metabolic activity was carried out by gas-liquid chromatography (GLC) in the Department of Gastroenterology FSBI "Academic Methodological Center Presidential Management Department of the Russian Federation"(MD, professor Ardatskaya M.D.) by method of Ikonnikov N.S., Ardatskaya M.D. et al. ("Method of separating a mixture of fatty acid, fraction C2 -C7, by GLC", Patent RU 2145511, cl. B 01 D 15/08, 20.02.2000). Immunological methods. Immunological studies were performed in the laboratory of immunological study methods, FSBI "Research Institute of Vaccines and Sera named after I.I.Mechnikov", RAMS (Chief of the Laboratory Ph.D., Professor Krasnoproshina L.I.). Lymphocyte CD3 +, CD4 +, CD8 +, CD16 +, CD72 + level was determined by flow cytofluorometry on a cytometer FAC Scan (BectonDickinson, USA) using monoclonal antibody of the same firm for differentiation and activation markers. To determine concentration of immunoglobulins IgA, IgG, IgM, method of radial immunodiffusion in the agarose gel sensu G. Manchini (1965) was used in the in the modification of E.V. Chernohvostova and G.P. German. Neutrophil phagocytic rate was determined by their absorbing capacity of suspension of killed Staphylococcus aureus. To examine the status of mucosal immunity, determination of concentration of immunoglobulins G, A and secretory A was determined in the saliva and coprofiltrates by immunodiffusion reaction sensu . Manchini (1965) in the in the modification of E.V. Chernohvostova and G.P. German using a commercial kit issued by Scientific and Production Center "Medical immunology". Study of IgE in the coprofiltrates was performed by ELISA using diagnostic kits "Diaplus", Scientific Production Association "Biotechnology" (Russia). Assessment of cytokine status included determination of serum levels major proinflammatory markers, IFN-γ and IL-1β, as well as anti-inflammatory IL-4 by ELISA assay using diagnostic test-systems R&D DiagnosticsInc. (USA). For approximate estimation of Th1 / Th2 ratio we used IFN-γ / IL-4 [Mosmann T., Sad S., 1996]. Allergology methods. General IgE in blood serum was determined by ELISA using a commercial test system released by LLC "TsKFF" (Stavropol). To determine the level of specific IgE antibodies to 5 groups of casually significant allergens, original techniques were used developed in the Laboratory of allergic diagnostics, FSBI "Research Institute of Vaccines and Sera named after I.I. Mechnikov", RAMS (Chief of the Laboratory Ph.D., Professor Gervaziyeva V.B.). Integral evaluation of sensitization was carried out using allergization index [Kobets T.V. et al., 2002]. Instrumental studies. Status of the liver, pancreas, gall bladder and stomach was evaluated by ultrasound method using diagnostic tool company «Aloca» (Japan) using convexion and linear array probe with a frequency of 3.5 MHz (by method of I.V.Dvoryakovsky, V.V. Lukin, L.V. Kedik, 1993). The study was carried out by Kozhevnikova E.N., MD and Ploskireva A.A., MD, specialists of ultrasound diagnostics, National public institution of health care Hospital for the infectious diseases of children №5, Moscow Department of health Methods and scope of laboratory and instrumental investigations are presented in Table1. Table 1. Methods and scope of the studies performed Study method Material Number of patients Number of studies Clinical methods Complete blood count Biochemical blood assay Blood acid-base balance Clinical urinalysis Coprological feces blood 476 634 blood 117 169 blood 81 152 urine 348 348 feces 81 143 feces 85 170 feces 476 476 scraping from anal 476 476 examination Biochemical feces analysis (microflora metabolites, free carbohydrates) Stool ova & parasites test Scraping for enterobiasis folds Immunological methods PHA (dysentery, blood 113 162 feces 314 449 nasal discharge 96 96 кровь 161 240 blood 79 158 blood 161 376 blood 47 94 saliva 47 94 feces 70 140 salmonellosis and other) Feces analysis for rotavirus antigen Nasopharyngeal swab for viruses (flu, parainfluenza, RS-viruses, adenoviruses) (ELISA) Immunological status, including cell (CD3+, CD4+, CD8+, CD16+, CD72+), humoral (Ig A, G, M) and natural (NKcells, neutrophil phagocytic rate, ФИ) immunity Cytokine status (IFN-γ, IL1β, IL-4) Allergological status General IgE Specific IgE to 5 antigen groups Mucosal immunity Ig G, A, secretory Ig A Secretory Ig A and Ig E Microbiological methods Feces bacterial feces 476 639 examination Washing bacteriological washings 78 78 feces 45 45 feces 141 282 inoculation Bacteriological inoculation for campylobacters Feces for dysbacteriosis by method of R.V.EpsteinF.l.Vilshanskaya Instrumental methods Abdominal ultrasound ECG 93 109 27 35 Other methods Molecular and genetic feces 357 436 methods Medical documentation Disease stories (from 003/ u) Inpatient cartograms 476 3640 Statistical analysis was performed using commercial software package Primer of Biostatistics 4.03 and statistical module of Microsoft Excel 2010 on the PC. Determined were the following values: statistical series percentage (%), simple average (M), standard deviation (STD), average error of the mean value (m). Comparison of the frequency of occurrence of qualitative clinical signs was based on comparison of empirical distributions using the χ2 criterion. Differences were considered significant for p <0.05, highly significant for p <0.001, insignificant for p> 0.05. The significance of differences between mean values was determined using Student's ttest (t). Differences were considered significant for p <0.05, highly significant for p <0.001, insignificant for p> 0.05. Results and discussion Structure of concomitant somatic pathology in children with acute enteric infections. Structural analysis of the concomitant somatic pathology in AII children admitted to the hospital, was conducted in 3640 patients in the period from 2002-2010. It has been established that allergic diseases accompanied AII in 1228 children (33.7%), dominating in frequency of occurrence in the major groups of somatic diseases of all age groups. In the second place were disease of the central nervous system (20%). The third place was occupied by diseases of the digestive system (14.4%) and ORT pathologies (13.1%). The smaller number of children experienced iron deficiency anemia (5.7%) and pathology of the genitourinary system (2.0%). The most frequent cases were AD (in 19.8% of patients with acute intestinal infections) and gastrointestinal food allergy (11.2%). The frequency of these diseases was highest in infants (25.2 and 11.8%, respectively) and tender age groups (18.2 and 11.7%, respectively). Allergic airway diseases were recorded in children of preschool and increased age (7.2 and 16.7%). In the structure of patients allergic diseases in the examined, food allergy and AD were determined in 58.7%, drug allergy - in 14.8%, airway diseases – in 7.1% (including bronchial asthma- 4.3%, allergic rhinitis- 3.4%). Thus, allergic diseases are the most common comorbidity in children with acute intestinal infections of, especially in infancy and tender age. These data confirm the results of studies conducted by Elezova L.I., (2006), Bitiyeva R.L. (2007), Gurieva O.A. (2010) on high incidence of concomitant allergic pathology in this category of patients. Clinical and laboratory features of acute intestinal infections in children with concomitant atopic dermatitis. To assess AD impact on the course and outcome of acute intestinal infections in children considering age, etiopathogenic variant of intestinal infection, type and severity of atopic dermatitis, comparison of clinical data and laboratory examination was conducted in comparable groups of 231 patients (study group) and 245 patients without allergic diseases (control group). It was established that in patients with atopic dermatitis acute intestinal infections are characterized by more frequent acute onset, with the development of basic clinical symptoms on the first day of the disease (in 85.3% of patients versus 72.7% in the comparison group (p <0.05), high frequency of moderate (67.1 vs. 53.9%, p <0.05) to severe forms (22.9 vs. 14.3%, p <0.01). Significantly more frequent in these patients was undulation (26.8 vs. 15.1%, p <0.001) and chronic (13.0 versus 4.5%, p <0.001) AII course, compared with children without allergic diseases for which a smooth course of the disease was typical (80.4 vs 60.2%, p <0.001). AII in the two comparison groups was often presented by gastroenteritis (41.1 and 46.1%, p> 0.01) and gastroenterocolitis (22.5 and 21.2%, p> 0.01). At the same time, children with AD experienced enterocolitis form of the disease significantly more frequent than those in the comparison group - 22.9 vs 13.9% (p <0.001). In our opinion, a significantly higher frequency of infectious diseases of the colon in the study group was due to the background allergic disease and was not associated with the disease etiology which can be confirmed by the results of scoring etiological causes of AII (see Fig. 1). Data presented in Figure 1. show no statistically significant differences in the etiological AII structure between the groups: rotaviruses and other diarrheal viruses (total percentage 61.4 and 60% in the main group and the reference groups, respectively) dominated, among the bacterial pathogens (total percentage - 21.9 and 25.9%, respectively) the most often was salmonella (9.8 and 11.9%, respectively), and AII frequency of combined etiology was 17 and 14.8% of which coincides with previously published data [Kozina G.A., 2009; Grigorovich M.S., 2011]. Fig. 1 Etiological structure of AII scored in the two groups (%) Figure captions: < Children with atopic dermatitis Rotaviruses 38.0 Other viruses 23.4 Salmonella 9.8 Shigella 4.3 Campylobacters 3.3 Children without atopic dermatitis Rotaviruses 40.0 Other viruses 19.6 Salmonella 11.9 Shigella 3.8 Campylobacters 4.3 Combined infections 17.0 Colibacillosis 4.5 Combined infections 14.8 Colibacillosis 5.1 Clinical performance of acute intestinal infections in the two groups was characterized by combination of intoxication symptoms (fever, apathy, loss of appetite) and dyspeptic manifestations (vomiting, diarrhea). At the same time, the children with concomitant AD experienced some differences. For example, statistically more significant was accidence of decreased appetite (67.5 vs 56.7, p <0.05), anxiety (50.6 vs 36.7%, p <0.05), and excitation (29.4 vs. 9.4%, p <0.05) in comparison with the control group. Fever was present in the majority of patients, and in 47.6-49 % of cases, it was pronounced. Presence of background allergy contributed to lengthening of febrile period (see Fig. 2) and increase in the number of patients who experienced persistent fever for more than 6 days (13 vs 3.3%, respectively, p <0.05). Fig. 2 The average duration of the main AII symptoms in the two groups of patients (* - Statistically significant differences in p <0.05). Figure captions: Comparison group Study group Diarrhea Vomiting Fever Loss of appetite Apathy Among dyspeptic symptoms of the disease the greatest differences were observed in the character, nature and duration of diarrhea syndrome. Up to 75.8% of the study group (p <0.05) experienced pronounced diarrheal syndrome (stool frequency higher than 6 times a day), and every third child – loose stool more than 10 times per day. In the comparison group, in contrast, 43.7% experienced moderate diarrhea (up to 5 times a day), and only 19.6% children (p <0.05) – multiple diarrhea. The mean duration of diarrhea syndrome in patients with concomitant AD was 1.8 days higher than in the control group (p <0.05) (Fig. 2). In the main group of patients diarrhea was significantly more seldom in comparison with the patients without AD, it was stopped during the first five days of the disease (28.1 vs 40.8%, p <0.05), and in 22.9% patients it persisted 8 days or more (comparison group – in 12.2%, p <0.05). it is noticeable that high frequency of blood admixture in stool (20.3 vs 11.4%, p <0.05), as well as watery diarrhea (30.7 vs. 14.7%, p <0.05) was determined in children with concomitant AD. Intensity and duration of vomiting had no significant difference between the patient groups. Significantly more frequent in the study group were symptoms of water and electrolyte imbalance (27.7 vs 16.3%, p <0.05), however the ratio of patients with second degree exsicosis did not differ significantly in the two groups (11.3 vs 9%, p> 0.05). It was established that scatological signs of exocrine pancreas insufficiency syndrome and dysbiosis in the acute AII period in children with concomitant AD was recorded significantly more often (67 and 82%) than in the comparison group (41 and 61%, p <0.05). In the late convalescence period (day 21-24 from the onset of the disease), 59% children with AD (p <0.01) still experienced signs of scatological syndrome, dysbiosis, 48% (p <0.01) - exocrine pancreas insufficiency, 37% - inflammatory changes, which can suggest about the negative impact of AD on the dynamics of normalization of scatological disorders. In the comparison group in these period scatological violations stopped in 57% children. Age features of acute intestinal infections in children with comorbid AD were studied in 3 groups of children. Children in the age under 1 year (n = 77) were in the group 1, 1 - 3 years (n = 60) – group 2, older than 3 years (n = 94) – group 3. The groups were comparable in admission terms and preclinical background. In comparative evaluation of infectious process features (see Fig. 3), there was no difference in the nature of the disease onset (in the vast majority of patients it was acute) and its course. Fig. 3 Infection process characteristics (%). Figure captions: Onset Under 1 year – 1 to 3 years – Older than 3 years Acute - subacute Course Disease topology Severity Under 1 year – 1 to 3 Under 1 year – 1 to 3 Under 1 year – 1 to 3 years – Older than 3 years – Older than 3 years – Older than 3 years years years Acute – ondulation - Gastroenteritis – Mild – moderate - chronic gastroenterocolitis - severe enterocolitis Gastroenteritis / enteritis prevailed among gastrointestinal lesions of all age groups. In this case, the frequency of enterocolitis in children aged 1-3 years was significantly higher than in the other groups (p <0.05), and gastroenterocolitis was recorded significantly more often in children of 1-3 years of age and older than in infants (p <0.05). On analyzing severity, a significantly greater frequency of severe AII can be noted in infants (24.7 - 30%). Comparative clinical characteristics of acute intestinal infections in children with AD in different age groups is shown on the Figure 4. Figure 4. Comparative clinical characteristics of acute intestinal infections in children with atopic dermatitis considering the age. Figure captions: < Under 1 year – 1 to 3 years – Older than 3 years Apathy – Loss of appetite – Anxiety – t 37-38°C – t 38.1 - 39°C – t=> 39.1°C – first degree exsicosis – second degree exsicosis – vomiting 1-2 times – vomiting 3-4 times – stool up to 5 times – stool 6-9 times – stool >10 times – hepatomegalia – megalosplenia> Manifestations of infection process common for all age groups of patients included loss of appetite, single vomiting, pronounced diarrheal syndrome (stool frequency in 27.7 - 36.4% patients was >10 times a day). At the same time a number of age-related features have been identified. Compared with other age groups, infants more often experienced anxiety, subfebrile fever, vomiting 3-4 times a day and hepatomegaly. In the patients of 1-3 years of age, AII clinical performance often included apathy, febrile fever, splenomegaly and symptoms of exsicosis (first and second degree). Comprehensive analysis of clinical data, anamnesis, results of standard and optional allergen-immunological surveys allowed to detect gastrointestinal forms of food allergy (GIFA) in 11.3% infants initially hospitalized with preliminary diagnosis of acute infectious enteritis / enterocolitis. In all the cases infectious etiology of the disease has been denied according to results of bacteriological, immunological, molecular genetic methods of activator identification. In the clinical performance, exacerbation of cutaneous manifestations of allergy was conspicuous, preceding onset of intestinal dysfunction, in particular liquid stool, loss of appetite, one third of the patients experienced low-grade fever without vomiting. GIFA diagnosis was confirmed by laboratory parameters, including high level of eosinophils in the peripheral blood (> 300μ / l) and in the feces, total IgE, detection of specific IgE and IgG4 antibodies to cow milk, goat milk and soya proteins. Standard basic therapy had no effect. An allergist performed further follow-up and treatment. One of the key factors determining the course and outcome of intestinal infection was a pathogenic disease variant depending on the pathogen type and pathogenicity factors. We have studied AD impact on clinical manifestations and course of the “watery” (n = 126) and "invasive" AII (n = 105). Course of "watery" AII type with AD background was characterized by higher frequency of acute onset (81 vs 64.3%, p <0.05), severity (moderate – 73 vs 61.1%, p <0.05) as well as undulating course in 26.2% (in the comparison group – 9.5%, p <0.05). Higher frequency and severity of intoxication symptoms (loss of appetite - 62.7 vs. 46%, p <0.05), general anxiety in every fourth child (in the comparison group – 10.3%, p <0.05), as well as anxiety (54 vs 38.1%, p <0.05) were main clinical symptoms in children with atopic dermatitis. Febrile fever was significantly more frequent in the comparison group (36.5 vs 23%, p <0.05), while its average duration had no significant differences (3.2 days in the main group compared to 3.7 in the comparison group, p = 0.08). Water and electrolyte imbalance was significantly more frequent in children with concomitant AD (44.5 vs 27.8%, p <0.05), which was due to pronounced diarrhea syndrome (stool >10 times a day in 32.5% vs 16.7% in the comparison group, "watery" type in 52.3% patients - (in comparison group - 27%, p <0.001). Diarrhea in AD patients resisted 1.5 days longer than in the comparison group (6.8 vs 5.3 days, p <0.001). When monitoring the dynamics of free carbohydrates in the feces, a phenomenon of lactase deficiency (LD) in the first days of the disease in the main group patients with recorded significantly more frequently than in the control group (86.5 vs 63%, p <0.05). At the same time, pronounced LD (level of carbohydrates in the feces higher than 1.1%) occurred 3.6 times more frequently in the study group than in the comparison group (13.5 vs 3.7%, p <0.05), and moderate LD – 1.3 times (43.2 vs 33.3%, p> 0.05). In the recovery period (days 21-24 of the disease,) moderate and high levels of carbohydrates in feces were determined in 27% patients of the main group (absent in the comparison group), in 43.3% minimum LD degree was recorded (37.5 in the comparison group). The data received show a slow recovery of enterocytes disaccharidase activity in children with concomitant AD, as well as possibility of hidden lactose intolerance. AII of "invasive" type in children with atopic dermatitis was characterized by more severe concomitant infection process (every third patient experiences a severe disease course, 60% patients - moderate (in comparison group – in 19% and 44.8%, respectively, p <0.05)), while there was an undulation in 27.6% (vs 21.6%) or a chronic disease (18.1 vs 6.7%, p <0.05). According to modern concepts, bacterial pathogens are primarily etiologic factors of invasive diarrhea. According to our data (see Fig. 5), in children without concomitant allergic diseases in 78% cases were allocated bacterial pathogens (Salmonella, Shigella, enteroinvasive Escherichia, Campylobacter, opportunistic microorganisms) and only in 22% - bacterial and viral association. In atopic children with clinical invasive diarrhea, frequency of mixed infections was significantly higher (36.5%, p <0.05), which may be caused by defects of mucosal immunity as well as deeper and communication of gastrointestinal lesions on account of concomitant allergic process in acute intestinal infection. Figure 5. Etiological structure of scored "invasive" type AII (* - Statistically significant differences for p <0.05). Figure captions: <Main group Comparison group Viral and bacterial mixt – Salmonella – Shigella – enteroinvasive – Opportunistic pathologic microorganisms - Campylobacter Comparative analysis of clinical symptoms in the acute period if invasive AII in children with and without AD allowed to establish significant differences in general anxiety frequency (35.2 vs 8.6%, p <0.05), timing of fever relief (4.8 ± 2.1 vs 3.9 ± 1.5 days, p = 0.002) and diarrhea (9.4 ± 1.8 ± 1.7 vs 7.6 days, p <0.001), as well as severity of diarrheal syndrome (loose stools more than 6 times a day, significantly more frequent than in the comparison group - 68.6 and 52.3%, respectively (p <0.01). Figure 6. Dynamics of scatological signs of inflammation in invasive AII Figure captions: <Mucus – Leukocytes: single – up to 20 per field – 20-50 per field – Eosinophils – Erythrocytes: single – up to 20 per field Main group: acute period – days 7-10 of the disease – days 21-24 of the disease Comparison group: acute period – days 7-10 of the disease – days 21-24 of the disease > To assess the relief of inflammatory process in the gastrointestinal tract, analysis of the main scatological values in "invasive" AII type was conducted in two groups. As seen on Figure 6, in AD children inflammatory changes were more persistent. Thus, by the period of early recovery macroscopic mucus persisted in 47.8% (comparison group – 22.2%, p <0.001), in all the patients, white blood cells were present in the feces, in 91.3% - in a significant amount (in the comparison group - only in 44.5%, p <0.05). In the period of late convalescence, scatological signs of inflammation persisited in 34.8% of patients of the main group (mucus, white blood cells), which was significantly more frequent than in the comparison group – 11.1% (p = 0.072). Status of the immune system in AII children with concomitant atopic dermatitis depending on AII etiology and immunopathogenic variant of allergic disease As a result of the studies, data were obtained indicating that AII in children is accompanied by changes of cellular, humoral and innate immunity whose direction and severity was directly dependent on the presence of concomitant allergic pathology. Thus, in patients with concomitant AD, during the acute phase of AII an increase in quantitative helper-inductorlevel of immune response was observed, that was demonstrated by a relative increase in the mean CD3+ and CD+-lymphocyte levels as well as immunoregulatory index (IRI) on background of CD8+ cells reduction (see Fig. 7). In comparison group, statistically significant violations were detected only in the pool of CD8 + lymphocytes whose average absolute and relative amounts were significantly reduced in comparison with healthy children. Frequency analysis revealed deviations of immunological parameters in all patients of the main group and in 77.8% in the comparison group (p> 0.05). Significantly more frequent than in the control group, deviation of absolute lymphocyte count was determined in children with AD (70.7% vs 46.7, p = 0.006) and CD8 +-cells (77.6 versus 40%, p = 0.023). In the control group the most often detected was deviation of IRI (in 71.1% patients, significantly more frequent than in the main group (p = 0.05) and the relative amount of CD16 +-cells (in 64.4%, no significant differences with the main group). Figure 7. Parameters of systemic immunity in children with acute intestinal infections considering allergic status (converted value of healthy children is equal to 1). Figure captions: Children with AD Children without AD Lymphocytes, % - Lymphocytes, abs. – CD3, % - CD3, abs. – CD4, % - CD4, abs. – CD8, % CD8, abs. – IPI – CD72, % - CD72, abs. – CD16, % - CD16, abs. – NPR (neutrophil phagocytic rate) – PI (phagocytic index) Lymphocytes, % - Lymphocytes, abs. – CD3, % - CD3, abs. – CD4, % - CD4, abs. – CD8, % CD8, abs. – IPI – CD72, % - CD72, abs. – CD16, % - CD16, abs. – NPR (neutrophil phagocytic rate) – PI (phagocytic index) The study of humoral immunity showed significant reduction in the number of Blymphocytes in patients with concomitant AD (p <0.001), but levels of major immunoglobulin classes had no significant differences with the comparison group (except IgE). In the innate immune system during the acute AII phase patients with AD experienced significantly more severe disorders (deficiency of NK-cells (p = 0.01), reduced phagocytic activity of neutrophils and phagocytic index). Figure 8. Correlations of immunological parameters in AII children with atopic dermatitis (A) and without AD (B). Note: The solid line indicates strong correlations (r = 0.7-1, for p <0.01), stipple correlation of medium strength (r = 0.3-0.7, p <0.01). Figure captions: A) Leukocytes – lymphocytes, % - lymphocytes, 109 – CD3, % - CD4, 109 – CD8, % - CD8, 109 – CD72, % - CD72, 109 – CD16, % - CD16, 109 – phagocytosis – completeness – IgG – IgA – IgM – IgE total B) Leukocytes – lymphocytes, % - lymphocytes, 109 – CD3, % - CD4, 109 – CD8, % - CD8, 109 – CD72, % - CD72, 109 – CD16, % - CD16, 109 – phagocytosis – completeness – IgG – IgA – IgM – IgE total Correlation analysis of the immune status revealed both general and specific correlation (defined in only one of the groups) (see Fig. 8). In both groups of patients a direct correlation of leukocyte level with absolute level of population and subpopulation of lymphocytes (in the main group strong ties were determined (r = 0.783 - 0.886, p <0.01), in the comparison group – vice versa: medium strength (r = 0.383 - 0.685, p <0.01) as well as with total number of lymphocytes with CD3 +-cells and absolute level of CD4 + and CD8 + lymphocytes. At the same time, in the main group general relationship was detected between relative number of lymphocytes and the absolute number CD72 + - (r = 0.444, p <0.01), between absolute number of lymphocytes and NK cells (r = 0.316, p <0.01). In the comparison group, relationship data were missing. At the same time, IRI value in the study group, as was expected, had a strong direct relationship with CD4 + rate (r = 0.758, p <0.01), and negative - with level of CD8 +-cells (r = -0.781; -0.398, p <0.01). In the comparison group, IRI level correlated only with the percentage of CD8 + lymphocytes (r = -0.492, p <0.01). Correlations were identified between AII severity and level of CD4 + - (r = 0.725, p = 0.0021), CD8 + - (r = -0.813, p = 0.0043), CD16 + (NK)-cells (r = -0.459, p = 0.0054). Diarrhea duration directly correlated with the level of CD8 + - (r = -0.781, p = 0.007) and NK cells (r = -0.623, p = 0.0082). In the analysis relationship between humoral and cellular immunity, features observed only in AD children were detected. In particular, IgG concentration in the serum, in addition to relationship with level of B-lymphocytes, correlated with the rate of CD8 +-cells (r = 0.402, p <0.01) and IPI (r = -0.424, p <0.01). According to numerous studies, reduction of the rate of CD8 +- cells in children suffering from atopic dermatitis is observed mainly in the period of acute disease and is accompanied by reduction of the main immunoglobulin classes. Considering the above said, is possible to speak about immunological causes of AD exacerbation on the background of intestinal infection. Increase in IgA and IgM serum levels in the comparison group correlated with the percentage of CD72 +-cells (r = 0.425; 0.378, p <0.01), which fully corresponds to the modern concept of humoral response in infectious diseases. However, noteworthy is inverse relationship of IgA level and phagocytic activity of neutrophils (r = -0.391, p <0.01) in children with atopic dermatitis. Considering literature data on IgA insufficiency in AD children and data obtained on the reduced level of phagocytic activity of neutrophils in the initial AII period, it can be assumed that the upward trend in IgA levels in the first days of the disease is a compensatory response to the lack of phagocytic immunity in children with atopic dermatitis. During the study, patterns of immune response were evaluated in AII children with allergic and non-allergic form of atopic dermatitis. Among the patients examined 61 children (52.5%) had allergic AD form and 55 (47.5%) - non-allergic form. Results of comparison of immunological values with the data of AII patients without concomitant allergic diseases are present in the Table 2. Table 2 Mean values of immunity system in AII children with allergic and non-allergic AD Immunologic values Children with allergic Children with non- Children without AD AD (n=61) allergic AD (n=55) (n=45) Lymphocytes 35,56 ± 0,97# 37,98 ± 0,75* CD 3+, % 70,29 ± 0,88*# 64,35 ± 1,15* CD 4+, % 47,87 ± 1,03*# 42,10 ± 0,80* CD 8+, % 23,94 ± 0,83*# 23,35 ± 0,65 IPI 2,32 ± 0,14# 1,95 ± 0,07 CD 72+, % 12,97 ± 0,79*# 17,95 ± 1,02* CD 16+, % 12,47 ± 0,65*# 14,32 ± 0,64* 67,58 ± 1,82 67,82 ± 1,59 64,7 ± 1,75 5,1 ± 0,25* 5,9 ± 0,47* 8,79 ± 0,26 IgG, g/l 6,29 ± 0,46# 7,88 ± 0,39 IgA, g/l 0,58 ± 0,02# 0,81 ± 0,06 1,30 ± 0,07 1,51 ± 0,09 383 ± 73,61*# 71 ± 2,07 NPR PI IgM, g/l IgE total, IU/l 39,1 ± 1,9 55,9 ± 1,8 31,7 ± 1,1 25,1 ± 0,8 1,80 ± 0,8 21,2 ± 0,82 18,4 ± 0,91 8,2 ± 0,42 0,74 ± 0,04 1,28 ± 0,09 89 ± 11,6 Statistically significant differences, p <0.05: * - between groups of AD patients, # - with comparison group. As a result of the studies, it was found that immunological disorders developed in both groups of AD patients. In acute phase of infection there was a significant increase in rate of CD3 + - and CD4 + lymphocytes (p = 0.021, p = 0.008) and IPI (p = 0.083), with a significantly lower rate of CD8 + - (p = 0.002), CD72 + - (p = 0.027) and CD16 +-cells (p = 0.017) in patients with IgEmediated AD form, in comparison with the second subgroup. The levels of IgG, IgA and IgM serum antibodies were significantly reduced. Changes revealed coincide with data of I.O. Shestakova (2010) for a group of patients with allergic AD. Increase in number of CD4 + lymphocytes and, consequently, IPI and reduction of CD8 + - (Tc1) cells on the background of IgE significantly increased in this form of AD compared with the other group, shows an important role of type I hypersensitivity in the pathogenesis of intestinal infection. Compared with patients without concomitant AD, patients of the first subgroup experienced a significantly lower rate of lymphocytes (p = 0.015) and B cells (p = 0,002), absolute number of CD8 + - (p = 0.01) and CD16 +-cells (p = 0.019); simultaneously a significantly higher rate of CD3 +- and CD4 +-cells (p <0.001, <0.001), and IPI (p <0.05) was recorded. Parameters of humoral immunity in this subgroup were characterized by a moderate IgG and IgA deficiency, against the background of overproduction of class E reaginic antibodies, on retention of IgM level comparable with that of children without AD. In patients with non-IgE AD form, significant differences of immunological parameters from those in patients without concomitant allergic diseases included a significant increase in white blood cell count (p = 0.016), rate of CD3 + - (p <0.001) and CD4 +-cells (p <0.001), due to decrease in total rate of lymphocytes (p = 0.048). Thus, it is possible to state that the most pronounced immunological disorders in AII develop in children with IgE-mediated form of atopic dermatitis. Given the immunopathogenesis differences, data for immune system status in AII of viral and bacterial etiology were analyzed, with a background of atopic dermatitis. The results are shown in Table 3 and Table 4. It was established that during the acute phase of AII of viral etiology in children with atopic dermatitis helper-inductor version of the immune response is mainly formed, as evidenced by elevated levels of CD4 + lymphocytes and IPI. In constrast, in the comparison group shifts immune suppressor mechanism was observed that was manifested through decreased levels of CD3 + - (p <0.001), CD4 + lymphocytes (p <0.01) and CD4 / CD8 index (p <0.05). Table 3. Mean values of the immune system in AII of viral etiology in children of the comparison group Immunologic Children with AD (n=32) Children without AD (n=13) Healthy values Acute patients Acute period Recovery Recovery (n=20) period CD 3+, % CD 4+, % CD 8+, % IPI 54,86±1,43** 52±1,6*,** 41,03±1,43*,** 39,4±1,3*,** 47,10±0,94* 44,6±1,2* 27,40±1,10* 25,6±1,39*,# 22,16±1,32 19,1±1*,** 21,30±1,20 1,97±0,16** 2,06±0,21** 1,29±0,09* 59,2±1,6 34,1±1,3 23,9±1,49 24,7±1,12 1,07±0,1* 1,6±0,1 CD 72+, % 20,19±2,60 22,3±1,3 19,20±1,10 24,90±1,70*,# 20,5±0,64 19,34±1,42 16,7±0,9** 18,50±0,90 20,70±1,23 17,5±1, 1 63,70±3,10* 59,4±2,7*,** 61,20±2,70* 70,2±1,80# 71,3±1,4 6,90±0,42*,** 7,80±0,74*,** 8,90±0,31* 8,74±1,64 6,3±1,3 0,70±0,16 0,96±0,19 (B-lymph.) CD 16+, % (NK-cells) NPR, % PI IgG, g/l IgA, g/l IgM, g/l 10,10±0,92 11,3±0,34 8,55±1,74 7,1±0,9 7,4±0,5 0,5±0,17 0,53±0,21 0,62±0,13 0,65±0,07 0,71±0,4 1,3±0,34 1,1±0,27 0,86±0,15 Note: Here and in Table 3 statistically significant are the following differences (for p <0.05, <0.001): * - with values for healthy children ** - with values of the comparison group, # - with values for the acute period. Parameters of humoral immunity in the two groups had no significant differences from those of healthy children. In the acute phase of viral diarrhea signs of failure of the innate immunity were determined, which manifested itself through a decrease in the neutrophil phagocytic activity and phagocytic index (mostly in children with concomitant AD) .On this background there was a compensatory increase in average level of NK cells in the two groups. In the convalescence period, a further increase of identified immune disorders was observed in AD children: in the dynamics - decreased levels of CD3 + (p <0.05), CD4 + - and CD8 + lymphocytes in the blood (p <0.01, p <0.01), as well as number of NK-cells (p <0.001), FAN (p <0.01). In the comparison group, a decrease the relative level of CD4 + lymphocytes (p <0.01) was detected, with no significant dynamics of the content of CD3 + - and CD8 +-cells, with simultaneous regeneration of functional activity of phagocytic immunity (NPR and PI values had no significant difference from physiological norm). The differences found in the dynamics of the immunological parameters allow to suggest about more persistent immunological disorders in intestinal viral infections, with the background of atopic dermatitis. Study of the state of the immune system in bacterial intestinal infections, given the presence of concomitant allergic diseases allowed to identify significant differences in the patterns of immune response between the groups compared (Table 4). Table4. Mean values of the immune system in AII of bacterial etiology in children of the groups compared Immunologic Children with AD (n=32) Children without AD (n=13) Healthy values Acute period Acute patients Recovery Recovery (n=20) period 71,83±1,38*,** 53,60±0,98*,# 56,20±1,50 54,70±1,12* CD 4+, % 46,73±1,67*,** 36,80±1,18**,# 30,10±1,35* 29,70±1,54* 34,1±1,3 CD 8+, % 21,58±1,20,** 22,70±1,60 27,90±1,40 21,50±1,20# 24,7±1,12 IPI 2,41±0,21*,** 1,62±0,15# 1,10±0,13* 1,38±0,23 1,6±0,1 25,70±1,67* 20,5±0,64 18,70±0,92 17,5±1, 1 CD 3+, % CD 72+, % (B- 14,66±1,16*,** 18,20±1,60** 59,2±1,6 22,70±1,50 lymph.) CD 16+, % 12,21±0,82*,** 15,90±1,11# 21,40±1,20* 64,20±3,40 63,50±2,40* 67,50±2,90 71,3±1,4 5,60±0,41*,** 7,80±0,57*,**,# 7,40±0,38* 9,80±0,67# 11.3±0.34 7,02±0,47** 9,80±0,74*,**,# 10,40±0,53* 12,40±0,50*,# 7,4±0,5 0,84±0,08* 0,67±0,12 0,86±0,04* 0,58±0,06# 0,65±0,07 1,54±0,16* 1,10±0,29 1,20±0,09 1,37±0,17* 0,86±0,15 (NK-cells) 66,16±3,99 NPR, % PI IgG, g/l IgA, g/l IgM, g/l Note: Statistically significant are the following differences (for p <0.05, <0.001): * - with values for healthy children ** - with values of the comparison group, # - with values for the acute period. Against AD background, a significant increase in CD3 + lymphocytes (p <0.001), CD4 +-cells (p <0.001), IPI (p <0.001) has been detected in acute period of bacterial AII as well as deficiency of NK-cells (p <0.05) and disorder of the functional activity of phagocytes (PI value of was significantly lower than in the comparison group and healthy children). In the humoral defense system in children with atopic dermatitis, concomitant pronounced deficiency of B-lymphocytes and IgG titer was observed that was compensated by high IgM levels in the first days of the disease. It was only during convalescence (days 10-14 of the disease) that IgG level increased, with level of B cells significantly lower than in the control group. In the comparison group, by contrast, reduction of cell immunity was observed which was primarily demonstrated by low CD4 + lymphocytes (p <0.01) levels and IPI (both in comparison with AD patients and reference physiological values). Reaction of the innate immunity system to the introduction of the infectious agent was also opposite: compensatory increase in the level of natural killer cells, on the background of oppression of neutrophil functional activity (reduction of NPR and PI, the latter remaining significantly higher than in the main group). In the infectious process dynamics on AD background, suppression of cell component of immune system took place, as evidenced by reduction of CD3 + lymphocytes, CD4 +-cells and IPI to the physiological norm level. In children with allergy-burdened analysis, only reduction of CD8 + lymphocytes was observed, with trends towards increase of initially reduced immunoregulatory index. Levels of T-lymphocytes and CD4 +-cells remained unaltered. Preserved were the symptoms of phagocytic immunity deficiency (CD16 +cells deficiency, reduction of PI value). In order to study cytokine regulation of the immune response in AII pathogenesis of viral and bacterial etiology, occurring on AD background, serum levels of pro-inflammatory (IFN-γ, IL-1β) and antiinflammatory (IL-4) cytokines (K) were investigated. It was found that in the acute phase of viral intestinal infection in children without concomitant atopia, serum IFN-γ and IL-1β was 4.2 - 6 times significantly higher than in healthy children (Table. 5). Simultaneously hyper-IL-4-cytokinemia was recorded, which plays a key role in the regulation of antibody response; it shows simultaneous activation of both Th1 and Th2-types of immune response whose function is to ensure adequate immune response aimed at pathogen elimination. On days 7-10 of the disease the level of IL-1β significantly decreased, indicating a decrease in the inflammatory process, as well as IL-4 concentration. Serum levels of IFN-γ had no significant dynamics, significantly exceeding values of healthy children in the period of clinical recovery, proving the intensity of anti-infectious immunity and providing effective body sanitation against the pathogen as well as smooth flow of the disease [Zheleznikova G.V., 2007]. Dynamic growth of index values IFN-γ / IL-4 showed a shift of functional Th1 / Th2 balance towards Th1-type of immune response. Table 5. Mean values of serum interleukins in AII of viral etiology in the patients of the groups compared (M±m) Immunologic Children with AD (n=32) Children without AD (n=15) Reference values Acute period Acute values Recovery Recovery period IFN-γ, pcg/ml IL-1β, pcg/ml 39,2±4,9*,** 13,7±3,9**# 106,5±10,4* 98,5±8,1* 78,4±64,7 463,7±34,2**,# 276,2±41,7* 113,9±25,1# 0-25 <50 IL-4, pcg/ml IFN-γ/ IL-4, U 97,2±7,5*,** 83,7±9,3*,**, 63,7±6,2* 29±9,6# 0,71±0,06*,** 0,52±0,07*,** 1,67±0,13 3,4±0,7* 6-24 1-1.5 Note: Statistically significant are the following differences (for p <0.05, <0.001): * - with values for healthy children ** - with values of the comparison group, # - with values for the acute period. In patients with concomitant AD, cytokine profile values of acute phase had significant differences. Concentration of IL-4 produced by Th2-lymphocytes was significantly greater control values and the values in the comparison group for all disease periods, which also means an imbalance of immunoregulatory mechanisms in Th2 terms. Th1 K-IFN-γ and IL-1β- was significantly (p <0.05) lower than in patients without atopy in the first days of the disease. Convalescence period was marked by increase in IFN-γ deficiency, which shows deterioration of antiviral defense mechanisms. Considering the data of R.M. Haitov (2009), a 6fold increase in IL-1β concentration revealed in patients with atopy is a marker of increase in the severity of allergic inflammation on the background of viral infection. A significant decrease in the index of IFN-γ / IL-4 reflected further increase in priority of Th2-type immune response. Thus, the results show significant differences in phase regulation of antiviral response on the background of persistent allergic inflammation and in its absence. As a result of the study of CK levels in patients with AII of bacterial etiology regardless of allergic status (p> 0,05), IL-1β and IL-4 overproduction was detected in the acute stage of the disease (Table. 6). Taking into account different IFN-γ baseline, it is possible to talk about domination of Th2-type immune response in children with atopy in the early days of the disease, and vice versa, balance maintenance of Th1 / Th2-immunocytes in the comparison group. However, in the convalescence period in patients with concomitant AD immune response shift towards Th1-dominance type was observed, which was primarily confirmed by the increase in IFN-γ and reduction of IL-4concentration. In the comparison group, levels of the above said CK decreased simultaneously, reflecting persistence of the balanced Th1/Th2-immune response. Table 6. Mean values of serum interleukins in AII of bacterial etiology in the patients of the groups compared (M±m) Immunologic Children with AD (n=32) Children without AD (n=15) Reference values Acute period Recovery Acute Recovery values period IFN-γ, pcg/ml IL-1β, pcg/ml IL-4, pcg/ml IFN-γ/ IL-4, U 34,2 ± 4,4** 75,6 ± 5,7* 49,1 ± 3,4*,# 386,3 ± 24,2*,# 52,1 ± 3,8*,# 0,94±0,07 237,1 ± 21,8* 93,7 ± 3,4*,**,# 321,4 ± 18,5*,# 112,7 ± 13,4* 43,5 ± 5,7*,# 284,4 ± 26,7* 87,6 ± 6,5* ,30±0,05*,** 2,14±0,11*,**,# 0,86±0,08* 0-25 <50 6-24 1-1.5 Note: Statistically significant are the following differences (for p <0.05, <0.001): * - with values for healthy children ** - with values of the comparison group, # - with values for the acute period. During the study, state of system mucosal immunity has been studied in 70 children with AII background, considering concomitant allergic pathology. The results are shown in Table 7. Table 7. Mean values of GIT mucosal immunity in AII patients of the comparison groups (M ± m) Patien Coprofiltrate Parotid fluid t sIgA, µg/ml IgE, IU/ml sIgA, µg/ml IgA, µg/ml IgG, µg/ml group Acute Convale Acute Convale Acute Convale Acut Convale Acut Convale s period scence perio scence period scence e scence e scence d perio perio d d Main 119±8, 148±10, 53±3, 41±2,7* 223,4+ 242,5+3 22,1+ 35,4+6, 13,3+ 12,6+1, group 7*,** 7*,**,# 1*,** , ** 35,2** 2,4* 3,6* 2,5* Comp 325±1 461,5±1 13±1, 6,2±0,7 437,6± 324,4±3 43,1± 51,1±10 18,74 16,4±3, arison 6* 2*,# 4* 42,2* 6,4*,# 7,4* ±3,6 4* 4* (n=45) * group y 2* , (n=25) Health ,4 # 62±2,4 0,421 154,4±24 63,4±3,1 21,8±2,3 childr en Note: Statistically significant are the following differences (for p <0.05, <0.001): * - with values for healthy children ** - with values of the comparison group, # - with values for the acute period. (1 - Partsalis Е.М., Revyakina В.А. State of local immunity of digestive tract//Pediatrics. 1983; 12: 19-21). Functional state of the gastrointestinal mucosal immunity in AD children in AII acute period was characterized by low production of the AII protective factors. Despite the levels of secretory IgA (sIgA) in feces and saliva increased in comparison with reference values (p <0.001), they were significantly lower (p <0,05) with regard to comparison group (n = 25) in all disease periods, which may indicate the reaction retardation of the local immunity with inoculation of the pathogen as well as inhibitory effect of hyper-IL-4-cytokinemia characteristic of AD [Khaitov R.M., 2009] on switching immunoglobulin synthesis from IgE to IgA and its subsequent secretion. The study was conducted of IgE level in the feces of AII children, which showed its significant increase regardless of the background of allergic patients (Table. 7, Figure 9). Figure 9. Dynamics of IgE level in feces of AII children (IU / ml) Figure captions: <IU/ml Acute period: Children with AD allergic forms (n=26) - mild AII form (n=13) - AII severe form (n=8) Moderate to severe AII without AD (n=12) Convalescence: Children with non-AD allergic (n=19) - Moderate to severe AII (n=24) - mild AII form without AD (n=13)> The data obtained coincide with the results of I.G. Gussoeva (2008) and allow to suggest participation of an allergic component in the pathogenesis of GIT inflammation, as well as possible protective role IgE as a barrier to the pathogen penetration in the intestine mucous membrane following sIgA [Zheleznikova G.V., 2011]. IgE exceeded reference values to the fullest extent in patients with concomitant AD and first of all in IgE-mediated form (see Fig. 9). In both groups of patients, IgE content in feces was highest in the moderate form, with a tendency to decrease with the recovery. However, by convalescence no IgE normalization in feces occurred in any of the groups examined. Evaluation of allergic disposition values (total IgE serum level and sensitization index) on AII background was conducted considering the disease etiology, severity and nature of the disease. These results obtained are shown on Figure 10. Figure 10. Dynamics of total IgE serum level and allergization index in AII children. Note: “” - values of healthy children. Figure captions: <IU/ml Total IgE level: Children with AD – Children withour AD Acute period – convalescence – after three months Allergization index: U Children with AD – Children withour AD Acute period – convalescence – after three months> During AII height and convalescence, general IgE level raised in patients with concomitant AD (p <0.001) and in the comparison group (p <0.05), which may indicate involvement of allergic reactivity in AII pathogenesis. Data obtained allow to explain the high rate of allergic diseases in the outcome of an intestinal infection, which was indicated by M.S. Grigorovich (2011) in her study. Subsequent examination of children 3 months after the onset of the disease showed normalization of total IgE level in patients without concomitant allergic diseases, while maintaining significantly high titers in AD children. High IA values in all observation periods were marked in patients with concomitant AD and only during the early convalescence in the comparison group. Dynamics of total IgE in AII of viral and bacterial etiology in children suffering from AD had significant differences (see Fig. 11). High titers of total IgE in AII of bacterial etiology determined in the first days of the disease, significantly decreased by the period of early recovery (p <0,01) and remained on the similar level up to 3 months of follow-up. Figure 11. Dynamics of total IgE in AII of viral and bacterial diseases in children suffering from atopic dermatitis. Figure captions: <IU/ml Acute period – Convalescence – After 3 months AII of viral etiology – AII of bacterial etiology – healthy patients> Figure 11. Dynamics of total IgE in AII of various severity in children suffering from atopic dermatitis. Figure captions: <IU/ml Mild – severe – moderate – healthy> On the background of AII of viral etiology, vice versa, increase in total IgE titers was marked by days 7-10 of the disease (p <0.01), as well as up to 3 months of follow-up (p <0.05). The differences defined in the dynamics of reaginic antibodies are determined by cytokine regulation of the immune response, first of all by persistence of hyper-IL-4-cytokinemia during the convalescence period of viral diarrhea. Level of the body allergic disposition correlated with course severity of intestinal infection (see Fig. 12). The highest concentration of reaginic antibodies in the early days of the disease was recorded in severe AII (r = 0.834, p = 0.0013), exceeding the values of moderate and mild forms 3.2 and 5 times, respectively (p <0.001). By the convalescence period, total serum IgE in severe AII significantly decreased, but remained well above the reference values as well as those for mild and moderate forms (p <0.01). Later on, IgE levels preserved their downward trend, reaching values of patients with moderate AII form. Comparative analysis of total IgE levels depending on AII course showed that a significantly higher level in the early days of the disease directly correlated with the further protracted intestinal infection (r = 0.782, p = 0.0075) (Fig. 13). Figure 13. Dynamics of total IgE serum considering AII course in children on AD background. (* - p <0.05) Figure captions: <IU/ml Acute period – severe acute disease – protracted disease – convalescence> Thus, high levels of serum IgE in the acute period intestinal infection may serve as a predictor of prolonged course of infectious process. GIT microflora state in AII children with concomitant atopic dermatitis Numerous literature data indicate formation of dysbiotic GIT disorders in AII pathogenesis in children, negatively effecting the course and outcome of the disease. At the same time, microflora GIT disorders are consistently recorded in children with atopic dermatitis and other allergic diseases, while their course correlates with the severity [Maximov A., 1997]. Regularities of GIT microbiocenosis response to AII in children with comorbid AD are not yet fully studied. A study of composition and metabolic activity of intestinal microflora was conducted in 141 AII patients, including 96 children with concomitant AD. Apart from allergic burden, disorders of GIT microflora were detected in 100% patients in the acute infection period. Serious (III degree) microecological disorders were significantly more frequently detected in children with atopic dermatitis (35.4 vs. 17.8% in the control group, p <0.05), and first of all affected obligate microflora microorganisms involved in formation of colonization resistance. In AII patients with AD background, deficiency of lactobacilli (<106 CFU / g) (p <0.01, χ2 criterion) and bifidobacteria (<108 CFU / g) (p <0.01) was significantly more frequent than in the comparison group (Fig. 14). Deficiency of typical Escherichia coli was recorded 1.8 times more frequently in the study group (p <0.01) and was accompanied by formation of strains with altered enzymatic activity. In 2/3 of all the AD patients, hemolyzing E.coli were detected (differences with the comparison group significant, p <0.01, criterion χ2 ). At the same time, frequency of detection of lactose-negative E.coli in acute AII had no significant differences in the comparison groups. Deficiency or excessive growth of enterococci was 1.5 times more frequent among patients with AD. Fig. 14 Astrogramm of deviation frequency of basic representatives of the intestinal microflora from the reference values (%). Figure captions: <Children with AD (n=96) – Children with AD (n=45)> It should be noted that the most frequent changes of lacto- and bifidobacteria pool have been observed in infants with atopic dermatitis (in 78.3 and 69.6% patients their titers were below 106 log IU/g), somewhat less in older age groups, however, in any case they were recorded significantly more often than in the patients without concomitant allergic diseases. In 57.2% patients with concomitant atopic dermatitis in AII acute period, the following disorders of facultative microflora were detected: excessive growth of staphylococci in 38.5% of patients (average 5,65 ± 0,76 logCFU/g), clostridia- in 48.9% (average 6.35 ± 1.09 logCFU /g) and Candida fungi - in 35.4% (average 5.7 ± 1.4 logCFU / g). Frequency of detection of these microorganisms in children of the comparison group was 20, 24.4 and 15.6%, with titers being significantly lower – 5.12 ± 0.41 logCFU/g (p <0.05), 5.67 ± 0.61 logCFU /g (p <0.05) and 4.8 ± 1.2 logCFU /g (p <0.05), respectively. In the convalescence period, obligate microflora imbalance was still detected in children with AD. In 38.5% patients the number of lactic acid bacteria did not exceed 105 CFU/g, and the average level in this group was significantly lower than in the comparison group (p <0.01) (Fig. 15). The frequency of lactobacilli deficiency was highest in infants - 60.9%, being significantly lower in children older than 3 years (26.8%, p <0.05). In the convalescence period of AII, in 29.2% of patients with AD Bifidobacteria deficiency remained, including 52.2% infants (in 30.4%, the level of bifidobacteria was 102 -105 CFU/g). The data obtained indicate the low regeneration potential of bifidobacteria and lactoflora in infants suffering from AD, which proves absolute necessity of biocenosis-corrective therapy in this age group. Fig. 15. Dynamics of the main elements of obligate microflora. Figure captions: <Ig CFU/g AD+ - AD- - AD+ - AD- - AD+ - ADAcute period – ocnvalescence – reference value> In AD children in the period of convalescence, in 42.7% cases hemolyzing E.coli was identified repeatedly, including 60.9% infants and 50% children at the age of 1-3 years. These changes were detected significantly more frequently than in the comparison group (p <0.01, χ2 criterion). In children suffering from atopic dermatitis, overgrowth of staphylococci (in 1/3 of all thepatients, mean value 6.1 ± 1.13 logCFU/g), bacteria of Clostridium genus (in 34.4%), fungi of Candida genus (in 27.1% of patients, mean value 4.7 ± 1.5 logCFU/g) was registered repeatedly. At the same time, level of other opportunistic pathogens was significantly decreased (Fig. 14). In the comparison group, detection frequency of the most widespread elements of facultative microflora in our work reduced during the convalescence period, except Candida genus fungi. When comparing the severity of AII and depth of microecological disorders considering concomitant allergic diseases, significant differences were detected only in moderate forms of the disease: in children with AD, III degree dysbacteriosis was registered significantly more frequently (39.6% vs. 16%, p <0.05), significantly less frequent was I degree dysbacteriosis (22.6% versus 48% in the comparison group, p <0.01). Relationship of degree of microecological disorders with the AD severity was observed in AD patients. It has been established that patients with mild AD (II degree), dysbiotic disorders of moderate severity (52%) prevailed, AD of moderate severity correlate with degrees II and III (32.4 and 44.1%, respectively), severe course with III degree (66.7%). In the observation dynamics, dysbiotic disorders progressed mainly on the background of moderate and severe AD. There is an ongoing study of the role of opportunistic pathogens in AD pathogenesis. Given the findings on their frequent detection in all periods of intestinal infection on the background of atopic dermatitis, sensitization to antigens of Staphylococcus aureus, Escherichia coli, Candida albicans and Proteus was studied in 35 children with AD and 12 patients without allergic pathology. In the acute phase of acute intestinal infection IgE antibodies of class 2 and 3 (moderate and severe sensitization) to S.aureus (48.6 vs. 16.7% in the control group, p <0.05, criterion χ2) and C. albicans fungi (31.4 against 16.7%, p <0.05) were significantly more frequent in AD children. In the disease dynamics a trend towards increase in sensitization to S.aureus and C.albicans in children suffering from atopic dermatitis as well as in the comparison group. Efficiency of modern enterosorbents, probiotics and antihistamines in the treatment of intestinal infections and AII reabilitation in AD children. One of the main methods of etiopathogenic therapy of intestinal infections is enterosorption, the effectiveness of which has been repeatedly confirmed. At the same time a comprehensive evaluation of the efficiency of natural and synthetic enterosorbents AII occurring on the background of atopic dermatitis has not yet been conducted in children. Considering this, we have examined 99 children with atopic dermatitis treated with diosmectit (smectit, n = 53) or polymethylsiloxane polyhydrate (enterosgel, n = 46) in moderate to heavy AII as well as 26 patients who were not treated with enterosorbents (comparison group). It has been found that the use of enterosorbents in AII initial treatment in children suffering from atopic dermatitis significantly reduced duration of intoxication symptoms, fever (by 0.9 day in diosmectit group, p <0.05) (Table. 8), as well as leukocyte intoxication index (LII) (from 3.1 ± 0.2 to 0.82 ± 0.1 U in diosmectit group (p <0.05); from 2.7± 0.1 to 0.6 ± 0.1 U- in Enterosgel group (p <0.05)). Table 8. Mean duration of AII clinical manifestations depending on the therapy conducted Symptoms Intoxication, days Fever, days Vomiting, from the Main group, n=99 Comparison group Smecta (n=53) Enterosgel (n=46) (n=26) 3,1±0,4* 3,4±0,3* 4,1±0,4 2,8±0,4* 2,9±0,4 3,7±0,2 1,4±0,2 1,9±0,2 1,6±0,1 initiation of treatment Exsicosis I-II degree, 2,3±0,2 1,6±0,1*# 2,5±0,2 days from initiation of treatment 3,1±0,2* 2,8±0,1* 4,1±0,3 Total days 5,6±0,6* 5,6±0,5* 7,2±0,5 Days from 3,2±0,5* 3,4±0,4* 4,8±0,3 Bloating, days from initiation of treatment Diarrhea initiation of treatment Note: significant differences, p<0.05: * - with comparison group; # - with diosmectit group Duration of gastrointestinal disorders (diarrhea syndrome and bloating) on the background of treatment with natural or synthetic sorbents was significantly lower than in the comparison group. Smooth disease course, with reduction of most of the symptoms by day 5 of the treatment, was more frequently observed in the study group (in 78.5% and 79.3%, against 57.9% in the comparison group, 0.05 <p <0.01, Fisher's ratio test). After 5-7 days of treatment, complete clinical recovery was achieved in 73.6% of patients taking diosmectit, and 76.1% taking Enterosgel. In the remaining patients stool was unformed, from 3 to 6 times a day, with pathologic impurities, bloating was observed as well as loss of appetite. In the comparison group, the proportion of such patients was significantly higher - 43.4% versus 26.4 and 23.9% (p <0.05, Fisher's ratiop test). Duration of exsicosis symptoms significantly decreased in the subgroup of patients receiving synthetic enterosorbent (Enterosgel) (p <0.05, Student's t test). Analysis of the results of occasional bacterioexcretion / viral shedding after the treatment course showed that clearing efficiency of enterosorbents was comparable with their clinical efficiency and was 73.4% for bacterial AII pathogens and 80.6% - for viral pathogens. Clearing efficiency of standard therapy did not exceed 57.2% and 40%, respectively, but the differences between the groups were not statistically significant. Uncludind enterosorbents in AII treatment schedule was accompanied by statistically significant reduction (58-63%) in the frequency of AD exacerbation. Upon that, date of initiation of enterosorption treatment was of crucial importance: the early (days 1-2 of the disease) administration enterosorbents reduced exacerbation of atopic dermatitis till only 18.9 - 23.9% in the study group; when administrating enterosorbents on days 3-4 of the disease, frequency of AD exacerbation was significantly higher-32.1 - 32.6% and had no significant differences with the comparison group (38.5%). The treatment conducted was effective in 88.7 - 89.7%, among them, 60.8-67.9% showed complete clinical efficacy, which was characterized by reduction of the majority of pathological symptoms by the end of day 3 of hospital treatment. Treatment efficiency of patients in the comparison group did not exceed 81%, the full being 57.6%. At the moment another promising direction of AII therapy optimization is the use of probiotics, whose mechanisms of achieving therapeutic effect are not limited to regeneration of the balance of the normal microflora, but also include immunomodulatory effects on mucosal immunity, anti-inflammatory and anti-allergic activity. To determine the tactics of probiotics use in AD children with intestinal infections, clinical and laboratory evaluation of the effectiveness of monocomponent (Enterol - 15 patients, Probifor - 23 patients) and multicomponent probiotics (Acipol - 34 children, Linex - 20 patients). Comparison group consisted of 63 patients receiving standard treatment. The study revealed no significant differences in the duration of vomiting and exsicosis (Table. 9). Relief of intoxication symptoms (including apathy, restlessness or loss of appetite) in the shortest possible time was observed in subgroups treated with Probifor and Linex. Fever duration significantly decreased in patients treated with Linex, in comparison with basic therapy and Acipol therapy (p <0.05). Relief duration of diarrhea decreased by 1.3 - 2.2 days when applying Probifor, Linex and Enterol (differences with the comparison group and Acipol- p <0.05, Student's t test). Full normalization of stool by the time of discharge from the hospital was significantly different between Linex and Probifor subgroups and the comparison group (91.3% vs 71.5 and 85%, p <0.05, Fisher's test). In the other groups, rate of patients with unstable or mushy stool had no significant differences with the comparison group after the treatment. Table 9. Mean duration of AII clinical manifestations depending on the therapy conducted and clinical efiiciency Symptoms Main group, n=92 Acipol Probifor Linex (n=34) (n=23) (n=20) 1 2 3 Comparison Enterol (n=15) group (n=63) 4 Intoxication, days 3,5±0,4*,1 3,3±0,2*,1,4 4,2±0,3* 4,9±0,4 Fever, days Vomiting, 5,1±0,4* 4,8±0,4 2,9±0,4 3,7±0,2 Total days 3,1±0,2 2,6±0,3 1,4±0,2 1,7±0,2 1,3±0,3 Days from 1,6±0,2 1,7±0,1 2,3±0,2 2,1±0,2 2,5±0,3 2,7±0,3 2,3±0,2 2,1±0,2 2,5±0,3 from the initiation of treatment initiation of treatment Exsicosis Days from 2,7±0,2 initiation I-II of degree, days from treatment initiation of treatment Diarrhea Total days 6,9±0,4 6,0±0,3*1 5,6±0,5*1 5,9±0,4*1 7,6±0,5 Days from 5,2±0,3 4,5±0,4* 3,7±0,3*1 4,1±0,3*1 5,8±0,4 59 69,6# 80# 73,3# 54 Incomplete 29,4 21,8 15 20 23,8 Absent 8,7# 5# 6,7# 22.2 initiation of treatment Complete Clinical efficiency 11.6 Here and in Table 10: statistically significant differences, p<0.05: * - with pretreatment level; 1 - with comparison group; 2 – taking Probifor; 3 – taking Linex; 4 – taking Enterol. On analyzing effectiveness of the studied biocenosis-corrective probiotics (Table. 10), a statistically significant increase and normalization of bifidobacteria levels in the feces was detected after treatment in Probifor or Linex subgroups, lactoflora- in Acipol, Probifor and Linex subgroups. Contents of a typical E. coli significantly increased in all subgroups of patients receiving probiotics, to the greatest extent - in Enterol and Linex subgroups, while it almost did not change in the comparison group. Table 10. Dynamics of microorganisms of obligate intestinal microflora in AII patients in the two groups (lgCFU/g) Microflora Examinatio Main group, n=71 forms n terms Acipol Probifor Linex Enterol n group, (n=21) (n=17) (n=20) (n=13) n=25 7,1±0,32 8,0±0,46 7,5±0,36 7,9±0,41 7,4±0,45 8,1±0,522, 9,6±0,37*,1 9,17±0,41*, 8,6±0,37 Bifidobacteri Before treatment a After Lactobacteri a 7,9±0,43 treatment 3 Before 5,4±0,38 6,03±0,52 6,2±0,42 5,7±0,32 5,2±0,6 6,7±0,5*,3 7,07±0,38*, 7,61±0,3*,1 6,5±0,413 6,1±0,38 1 treatment After treatment E. coli Compariso Before 1 7,1±0,5* 6,1±0,39 6,6±0,28 6,1±0,52 6,3±0,41 7,7±0,371 7,36±0,47*, 7,93±0,37*, 8,08±0,62*, 6,7±0,31 1, 4 1 1 treatment After treatment The positive dynamics of facultative intestinal microflora was marked for use of Linex and Enterol in AII treatment in patients with AD. In the subgroup treated with Linex, frequency of detection of elevated titers of hemolyzing and lactose-negative E. coli, Staphylococcus, yestlike fungi and enterococci in feces significantly (2-3 times) decreased after treatment, and in only 10% of patients elevated levels opportunistic pathogens level persisited (Table. 11). On the background of Enterol therapy, 1.8-fold decrease was noted in the frequency of E. coli with modified enzyme activity, 2.3 times- of clostridia, 2.5 times – of elevated enterococci titers and 3 times – of staphylococci. However, despite the above-mentioned positive results of probiotic therapy, more than 20% children with AD, AII convalescents, continued to identify high concentrations of E.coli with altered enzymatic activity, yeast-like Candida fungi, Clostridia. Combined therapy of these patients should include, along with the probiotics studied, other biocenosis-corrective agents (prebiotics, enteral immunoglobulins etc.). Table 11. Frequency of compositional disorder of facultative intestinal microflora on the background of probiotic therapy in AII patients (%) Microflora Examination Main group, n=71 forms terms Acipol Comparison Probifor Linex (n=20) (n=21) (n=17) E. coli with changed enzyme activity, >105 Before Enterol group, (n=13) n=25 72 52,4 64.7 55 69,2 33,3 29.4 25 38,5 28,6 41.2 40 20,8 36 23,8 23.5 10 28.5 28 47,6 35.3 6,6±0,28 6,1±0,52 6,3±0,41 23,8 29.4 7,93±0,37*,1 8,08±0,62*,1 6,7±0,31 66.7 47,1 55 46,2 52 38,1 35.3 20 30.8 28 28,6 41.2 30 38.5 28 19 17,6 10 15.4 20 treatment After 52 treatment (↑) Staphylococcus, Before >103 (↑) treatment After treatment Сlostridium, Before >105 (↑) treatment After treatment Enterococcus, Before >25% treatment After treatment Opportunistic Before pathogens treatment (Proteus, After Citrobaсter, treatment Enterobaсter идр.), >104 (↑) Thus, in the complex initial AII treatment on the background of AD the most rational was the use of polycomponent probiotic Linex and drug Saccharomyces boulardii, which enhances clinical efficacy and normalization of the intestinal microflora in 60.5% patients. Given the evidence of a non-smooth AII course on the background of AD, including high frequency of acute allergic process in acute and convalescence period, a comparative evaluation was carried out on AHP efficiency in AII treatment on the background of moderate severity AD according to two schemes: I (n = 50) - basic therapy with I generation AHP (suprastin, tavegil, average course duration – 6.3 ± 1.7 days.) with subsequent transition to III generation AHP (zyrtec, average course – 12.7 ± 1.4 days); 2 (n = 37) - basic therapy with the inclusion of II generation AHP (zyrtec, average course duration – 17.8 ± 2.9 days.). Comparison group consisted of 47 patients not receiving AHP. Compared groups were matched in terms of hospitalization (76, 81.8, 80.6% - on days 1-3 of the disease), clinical variant (gastritis/gastroenteritis/enteritis - 62, 56.8, 61.7%, gastroenterocolitis / enterocolitis -38, 38.3, 43.2%), etiology (viral - 42.4, 40.5 and 44.7%, bacterial - 33, 35.1, 25.5%), as well as AD severity (mild - 34, 40.5 and 44.7%, average - 52, 45.9 and 51.1%; severe- 14, 13.5 and 8.5%, respectively). During the study, no significant difference in the duration of intoxication, vomiting and diarrhea syndrome was found (Table. 12). At the same time, abdominal pain was reduced in the shortest possible time on the background of II generation AHP, and the average rate of vomiting in patients receiving I generation AHP was significantly less than in the comparison group (2.4 vs 4.3 times/day, p <0.05). Table 12. Clinical values of AHP efficiency in the complex AII therapy on the background of atopic dermatitis. Value Intoxication duration, Standard therapy + AHP Standard therapy Schedule I (n=50) Schedule II (n=37) (n=47) 4,5±0,5 4,1±0,6 4,6±0,5 3,9±0,4 4,6±0,3 4,3±0,6 2,1±0,7 1,9±0,5 2,8±0,7 6,7±0,9 5,2±0,6 6,3±0,7 days Fever duration, days Vomiting duration, days Diarrhea duration, days Abdominal pains, 3,1±0,5 2,7±0,4# 4,3±0,5 14.5% 11.3% 26,3% 54* 48,6* 78.7 40,7* 50* 13,5 (n=27) (n=18) (n=37) 51,9* 38,9 22,9 (n=27) (n=18) (n=37) days Non-smooth AII course, % Increase in AD clinical manifestations in AII acute period, % AD clinical remission by the end of AHP course, % Decrease in AD clinical manifestations by the end of AHP course, % Notes: statistically significant differences with the comparison group, p<0.05: * Fischer’s test, # - Student’s t-test. Earlier AHP administration significantly reduced the frequency of AD exacerbations in acute phase of an intestinal infection. After treatment, the positive dynamics of AD course presented by clinical remission was recorded in half of the children treated with zyrtec (p <0.001), as well as in 40.7% receiving I generation AHP, with subsequent transition to II generation AHP. Thus, efficiency analysis of desensitizing drugs in the treatment of acute intestinal infections in AD children demonstrates the benefits of II generation AHP (zyrtec) from the perspective of ensuring a favorable course allergic process (reduction in the frequency of AD exacerbation in AII acute and convalescence period, decrease in severity of skin allergy and terms of its relief). Antihistamines do not have significant impact on the dynamics of AII gastrointestinal manifestations. Follow-up of 52 AD patients convalescent from AII without receiving no special rehabilitation remedies within 1-3 months, showed presence of persistent intestinal dysfunction in 32.7% of patients in the form of episodes of unstable stool, a tendency towards stool retention/ constipation (in 28.8%), loss of appetite (in 46.5%), complaints of abdominal pain (36.5%), pathological impurities in the stool. At the same time, increase in AD clinical manifestations was recorded in 48.1% children. Study of intestinal microbial homeostasis in 21 children has revealed presence of major dysbiotic violations (second degree – in 57.1%, third degree- in 33.3%), due to low level of obligate microflora (average number of bifidobacteria-7.8 ± 0.7 lgCFU/g, lactobacilli-6.3 ± 0.8 lgCFU/g), high titers of staphilococcus aureus (in 38.1%), Candida fungi (in 23.8%) and Clostridium bacteria (33.3%). Taking into account the results obtained as well as literature data, a rehabilitation complex was proposed for AII children on AD background, which included long-term (up to 3 months) course of II generation AHP, polycomponent probiotic (Linex) or probiotic products for functional nutrition (based on age, nature of gastrointestinal disorders and absence of allergy to cow milk protein). Efficiency qualification of the proposed complex was verified in the group of 72 children with AD suffering from AII. As a result of the complex, positive dynamics of GIT disorders was demonstrated in 70.8%, cutaneous manifestations in atopy – in 31.9%, frequency of expressed dysbiotic violations also decreased (by 41.7%), exacerbation of atopic dermatitis in the late convalescence period was observed in 26.4% (significantly less than in the comparison group – 48.1% (p <0.001)). CONCLUSIONS 1. In every third child hospitalized due to acute intestinal infection, a concomitant allergic pathology is recorded, at the top of which were atopic dermatitis and gastrointestinal food allergy (58.7%). Most commonly allergic diseases accompany acute intestinal infections in young children. 2. Manifestation of GIT form of food allergy in 11.3% children of first year of life is accompanied by clinical signs of acute intestinal infection that determines the need for additional allergen immunoassay for timely diagnosis and rational therapy. 3. Acute intestinal infections in children with concomitant atopic dermatitis are characterized by higher severity, manifestation and duration of the main clinical manifestations as well as undulating course in every fourth child. In the recovery period, scatological disorders, functional dyspepsia, lactase deficiency and changes in the microflora composition of metabolite status intestine more often identified and longer persisted in these patients. 4. Factors determining severity and course of acute adverse intestinal infections occurring on the background of atopy as well as slow body detoxification from the pathogen, are early age of the patients, IgE-mediated atopic dermatitis and its severe course. 5. Development of acute intestinal infections on the background of atopic dermatitis in children is accompanied by disturbances of the immune homeostasis. The deepest change in the parameters of cellular and humoral immunity is observed in IgE-mediated, severe variant of atopic dermatitis: in 77.5% patients, imbalance of T-cell immunity formed, with a significant increase in CD4 + lymphocytes and IRI, CD8 + lymphocytes and NK cells insufficiency. A correlation is determined between immunological disorders, AII severity and terms of relief of diarrheal syndrome. 6. In children with acute intestinal infection of viral etiology on the background of atopic dermatitis, predominance of Th2-immune response is marked, both in the acute period of infection and in convalescence. Increase in concentrations of IL-4 serum on the background of IFN-γ deficiency in the onset of the disease correlates with high frequency of its undulating and protracted course, as well as increase of sensitization level during the convalescence period. In the group of patients without concomitant allergic diseases, a balanced Th1/Th2immune response is observed in the acute period with a shift towards Th1-variant during convalescence. 7. In the acute phase of intestinal infections of bacterial etiology in children regardless of allergic pathologies, a shift in cytokine balance is observed towards Th1-immune response mediated by IFN-γ and IL-1β, on the background of constitutional hyper-IL-4-cytokinemia. In the convalescence period, a further increase is marked in the levels of pro-inflammatory mediators IL-1β and IFN-γ, along with reduction of IL-4. 8. In the convalescence period of viral intestinal infection in children with concomitant AD, an increase in the level of total IgE and specific IgE-antibodies to opportunistic pathogens is marked that is caused by persistence of dysbiotic disorders of intestinal microflora, lack of local immunity factors (sIgA) and prevalence of Th2-cytokine profile in the blood serum. In acute intestinal infection of bacterial etiology, level of Class E reaginic antibodies significantly decreased by the period of early convalescence and remained stable in the period of late convalescence. 9. Functional state of GIT mucosal immunity in children with atopic dermatitis in the acute phase of intestinal infection is characterized by low production of free and secretory IgA (2-2.5 times lower than in the absence of atopy) as well as a high level of IgE in coprofiltrate. 10. In all children with AII and AD, major violations of the intestinal microflora were revealed which correlated with age, etiology and severity of the underlying disease. On the background of atopic dermatitis, the most often was sensitization to some kinds of conditionally pathogenic microflora (specific IgEantibodies to St.aureus, C.albicans, E.coli, with altered enzymatic properties). 11. Initiation AII treatment in children with concomitant atopic dermatitis based on a graded administration of enterosorbents, probiotics and antihistamines, accelerates the recovery, improves disease prognosis and outcome, reduces exacerbation risk and duration of the background allergic disease as well as reduces severity of microecological intestinal disturbances. 12. The developed complex of rehabilitation measures allows to reduce the risk of post-infectious gastrointestinal disorders (by 70.8%) and AD exacerbation (by 41.7%) in the period of late convalescence. PRACTICE RECOMMENDATIONS 1. For the purpose of differential diagnostics of acute intestinal infections and manifestations of GIT food allergy in infants, it is advisable to take into account: • medical history - exacerbation of cutaneous allergy manifestations preceding appearance of dyspeptic symptoms; • clinical data - gradual onset of the disease, prevalence of enteritic and enterocolitic types of diarrheal syndrome, moderately severe intoxication symptoms (loss of appetite, subfebrile fever); • laboratory data - results of bacteriological, immunological, molecular genetic methods to identify pathogens, the level of eosinophils in peripheral blood and in the feces, total IgE, specific IgE and IgG-antibodies to cow milk proteins, gluten, goat milk and soy. 2. To monitor the course and outcomes of acute intestinal infections in children with concomitant atopic dermatitis in the early stages of the disease, it is recommended to use the following immunological parameters: total IgE of the blood serum (basal value > 300 IU/mL is a predictor criterion of protracted course), secretory IgA in coprofiltrate (basal value <50 mg/ml a predictor of non-smooth and heavy course of infection), IgE in coprofiltrates (baseline> 80 IU/mL is a predictor of common gastrointestinal lesions and severe disease). 3. For children with atopic dermatitis with acute intestinal infections receiving complex initial therapy, earlier administration of the following drugs is recommended: a) antihistamines – in children up to 6 months, in repeated vomiting - suprastin, tavegil (in age-related dosage), the rest of the patients - zyrtec (in age-related dosage); b) enterosorbents - in domination of diarrheal syndrome, presumably of viral etiology e – dioctahedral smectit (in age-related dosage), in case of expressed intoxication syndrome – polymethylsiloxane polyhydrate (in age dosage) in the course up to 10 days; c) probiotics - in advanced GIT lesions as well as in expected viral etiology of the disease (excluding presence of AD exacerbation) - Linex (course not less than 2 weeks); in expressed diarrheal syndrome and without AD exacerbation - Enterol (course not less than 7-10 days). 4. In the convalescence period of acute intestinal infections in children with concomitant atopic dermatitis, it is recommended: a) To continue desensitizing treatment with II generation AHP (Zyrtec) within three months; b) to correct microecological intestinal disturbances: • in the presence of allergy to cow milk protein as well as in children of early age repeated courses of polycomponent probiotics (up to 10 -14 days within 3 months); • in children older than 3 years -daily intake of probiotic products for functional nutrition with Lactobacillus casei DN-114001 (in persistent intestinal dysfunction) or Bifidobacterium animalis DN-173010 (with a prevalence of hypokinetic motor disorders). SCIENTIFIC PUBLICATIONS 1. Gorelov A.V., Milutin L.N., Usenko D.V. Tactics of the complex therapy of acute intestinal infections in children. Problems of Modern Pediatrics, 2003. № 2. S. 82-3.* 2. Usenko D.V., Matanina N.V., Milovanova S.V. Use of Saccharomyces boulardii in the treatment of intestinal infections in young children. Russian Medical Journal, 2003 Vol. 3. № 175. S. 218. 3. Usenko D.V. Acute enteric infections in children with atopy. Materials of XI Congress of Russian pediatric gastroenterologists "Actual problems of abdominal pathology in children ", Moscow, 2004, S.325-326. 4. Usenko D.V. Course of acute intestinal infections in children with atopic dermatitis. Materials of IX Congress of Russian pediatricians "Actual Problems of Pediatrics ". Moscow, 2004, S.425 5. Usenko D.V., Gorelov A.V. Use of elidel in treatment of atopic dermatitis in children. Materials of IX Congress of Russian pediatricians "Actual Problems of pediatrics", Moscow, 2004. P.426. 6. Elezova L.I., Usenko D.V., Gorelov A.V., Karasev E.A. New approaches to treatment of intestinal infections in children. Materials of scientific conference of Russian pediatricians "Pharmacotherapy in Pediatrics". Moscow, 2004, P.36. 7. Elezova L.I., Usenko D.V., Gorelov A.V. New approaches to correcting disturbances of intestinal microflora in acute diarrhea in children. Materials of scientific and practical Conference of Russian Pediatricians "Pharmacotherapy in Pediatrics". Moscow, 2004. P.37. 8. Usenko D.V., Elezova L.I., Gorelov A.V. Influence of probiotic Lactobacillus Casei DN 114001 on state of mucosal immunity in acute intestinal infections. Materials of scientific conference of Russian pediatricians "Pharmacotherapy in Pediatrics". M., 2004 - P.94. 9. Semenov B.F., Gorelov A.V., Usenko D.V., Elezova L.I. et al. Evaluation of probiotic foods in the complex treatment of acute intestinal infections in children. Infectious diseases, 2004. Volume 2, № 4. Pp.52-58. * 10. Usenko D.V., Elezova L.I., Gorelov A.V. Study of the effect of probiotic Lactobacillus casei DN-114001 on indicators of mucosal immunity in acute intestinal infections in children. Materials of Russian scientific and practical conference "Key problems of infection control". St. Petersburg, 2005, S. 11. Usenko D.V., Kadzhaeva E.P., Gorelov A.V. Clinical efficiency of nifuroxazide in the treatment of acute intestinal infections in children. Materials of Russian scientific and practical conference "Treatment of infectious diseases in children: modern conceptions and unsolved problems ", St. Petersburg, October 1113, 2005. S. 127. 12. Gorelov A.V., Usenko D.V., Elezova L.I., Ardatskaya M.D., Burkin A.V. Qualification of efficiency of probiotic product correction in microecological disorders in acute intestinal infections in children. Epidemiology and infectious diseases, 2005. №6. pp. 58-61. * 13. Usenko D.V., Burkin A.V., Elezova L.I., Gorelov A.V. et al. Evaluation of a new approach to dietary correction in acute intestinal infections in children. Epidemiology and infectious diseases, 2005. №5. pp. 41-43. * 14. Gorelov A.V., Usenko D.V., Elezova L.I., Ardatskaya M.D., Ivanchatenko G.G. Efficiency of probiotic product in correction of intestinal microflora disorders in children with acute intestinal infections. Problems of pediatric dietology, 2005 Vol. 3. №5. pp. 42-46.* 15. Elezova L.I., Kadzhaeva E.P., Gorelov A.V., Usenko D.V., Burkin A.V. Clinical and laboratory evaluation of the efficiency of traditional fermented diary products in dietary management of acute intestinal infections in children. Materials of Russian conference "Actual problems of pediatrics" - Oryol, March 30-31, 2005. P.54. 16. Usenko D.V., Gorelov A.V., Shabalin S.V. Use of probiotic products containing Lactobacillus casei defensis in treating intestinal infections in children with altered allergic reactivity. Infectious diseases, 2005 Vol. 3. № 3. pp. 51-55.* 17. Gorelov A.V., Usenko D.V., Ardatskaya M.D. Use of probiotic products for correcting microflora disturbances in acute intestinal infections in children. The attending physician, 2006. №7. p. 89. 18. Kadzhaeva E.P., Gorelov A.V., Usenko D.V., Bitiyeva R.L. et al. Etiologic structure of acute intestinal infections in children hospitalized in a large Moscow hospital. Infectious diseases, 2006 Vol. 4. № 2. pp. 34-36. * 19. Gorelov A.V., Usenko D.V., Kadzhaeva E.P., Ardatskaya M.D. Evaluation of clinical efficiency of enterofuril in complex therapy of acute intestinal infections in children and its effect on intestinal microbiocenosis. Infectious diseases, 2006 Vol. 4. №3. pp. 47-50. * 20. Gorelov A.V., Usenko D.V. Probiotics: mechanisms of action and efficacy in infections of the gastrointestinal tract. Epidemiology and Infectious Diseases, 2006. №4. pp. 53-57. * 21. Usenko D.V., Gorelov A.V., Pogorelov O.A. New horizons of probiotics application. Infectious diseases, 2006 Vol. 4. № 4. pp. 57-61. * 22. Gorelov A.V., Usenko D.V., Ardatskaya M.D. Biocenosis-saving efficiency of nifuroxaside with acute intestinal infections in children. Problems of modern pediatrics, 2007 Vol. 6. №2. pp. 110-114. * 23. Kadzhieva E.N., Usenko D.V., Gorelov A.V., Ardatskaya M.D. Modern nitrofurans in the treatment of intestinal infections in children. Farmatheca, 2007. № 13. pp. 79-82. 24. Gorelov A.V., Usenko D.V. Rotavirus infection in children. Problems of modern pediatrics, 2008. Vol. 7. № 6. pp. 78-84. * 25. Ukraintsev S.E., Gorelov A.V., Ardatskaya M.D., Usenko D.V. The dynamics of short-chain fatty acids spectrum in the feces of children with viral diarrhea on the background of application of probiotic mixture. Pediatrics. Journal named after G.N. Speransky, 2008 Vol. 87. №6. pp. 82-86. * 26. Gorelov A.V., Usenko D.V. The role of microflora in the gastrointestinal tract and principles of correctuion of disturbances of its composition. Russian Medical Journal, 2008 Vol. 16. №19. pp. 1-5. 27. Gorelov A.V., Usenko D.V., Elezova L.I. Combined therapy of acute intestinal infections in children. The attending physician, 2008. №4. p. 94-95. 28. Usenko D.V., Gorelov A.V., Shabalin S.V. Experience of using fermented milk probiotic product in the treatment of acute intestinal infections in children with atopic dermatitis. Pediatrics. Journal named after G.N. Speransky, 2008 Vol. 87. № 4. pp. 85-90. * 29. Gorelov A.V., Usenko D.V., Ardatskaya M.D., Zaguzova L.I., Trefilova I.Sh. Clinical and laboratory evaluation of the effectiveness of lactulose in the treatment of rotavirus infections in children. Infectious diseases, 2008 Vol. 6. №2. pp. 24-28. * 30. Usenko D.V., Gorelov A.V., Elezova L.I., Zaguzova L.I. et al. Comparative evaluation of efficiency of probiotic food and monostrain probiotic in AII children older than 3 years. Proceedings of the Conference dedicated to 50th anniversary of Department pediatric infectious disease of Moscow Regiobnal Research and Clinical Institut,. 2008. pp 51-54. 31. Gorelov A.V., Milutin L.N., Reyzis A.R., Usenko D.V., Nikitin T.S., Drondina A.K., Ploskireva A.A., Ruzhentsova T.A. Results and prospects of studying the problem of acute intestinal and respiratory infections and hepatitis in children. Epidemiology and infectious diseases, 2009. №2. pp. 51-57. * 32. Usenko D.V., Gorelov A.V. Lactase deficiency in children. Consilium medicum. Pediatrics, 2009. №1. pp. 33-36. 33. Barmina O.S., Gorelov A.V., Usenko D.V., Ardatskaya M.D. Clinical and laboratory efficiency of multiprobiotic drug Acipol in adjuvant therapy of "Invasive" AII in children. Infectious diseases, 2009 Vol. 7. № 1. pp. 76-79. * 34. Usenko D.V. Dietary correction of colon dysmotility. Attending physician, 2009. №7. pp. 82-84. 35. Parfenov A.I., Usenko D.V., Prilepskaya S.I. Use of probiotic products in the correction of mild digestive disorders. Pharmatheca. 2009. №2. pp. 76-79. * 36. Ploskireva A.A., Usenko D.V. Role of probiotics and probiotic products in treatment and prevention of infectious diseases. Infectious diseases, 2010 Vol. 8. №3. pp. 58-64. * 37. Usenko D.V., Shabalin S.V., Gorelova E.A. Infections and allergies. Infectious diseases, 2010 Vol. 8. № 2. pp. 68-74. * 38. Gorelov A.V., Ploskireva A.A., Usenko D.V., Tkhakushinov N.H. The modern approach to correction of exocrine pancreatic insufficiency syndrome and malabsorption in children with acute intestinal infections of viral etiology. Infectious diseases, 2010 Vol. 8. №2. pp. 89-92. * 39. Usenko D.V., Gorelov A.V., Shabalin S.V. Features of the cytokine profile in acute intestinal infections of viral etiology in children with atopic dermatitis. Materials of the III Annual Russian Congress on Infectious diseases. Moscow, March 27-29, 2011. - Infectious diseases, 2011 Vol.9, application 1, p.371 40. Usenko D.V., Gorelov A.V., Shabalin S.V., Gervazieva V.B. Dynamics of allergic reactivity values in children with acute intestinal infections. Materials of III Annual Russian Congress on Infectious Diseases. Moscow, March 27-29 2011. Infectious diseases, Vol.9. 2011, Annex 1, p.371-2. 41. Ploskireva A.A., Gorelov A.V., Usenko D.V., Bondarev A.V., Tkhakushinov N.H., Uluhanova L.U. Efficiency of a causal treatment of acute intestinal bacterial infections in children at the present stage. Infectious diseases, 2011 Vol. 9. №4. Pp. 79-83. * 42. Usenko D.V., Gorelov A.V., Samarin A.S., Shabalina S.V. Features of acute intestinal infections of viral etiology in children with atopic dermatitis. Materials of IV Russian Annual Congress on Infectious diseases. Moscow, March 26-28, 2012. Infectious diseases, Vol.10. 2012, annex 1, p. 396 43. Usenko D.V., Gorelov A.V., Shabalina S.V. Sensitization to antigens of conditionally pathogens in acute intestinal infections in children. Materials of IV Annual Russian Congress on Infectious Diseases. Moscow, March 26-28 2012. Infectious diseases, Vol.10. 2012, Annex 1, p. 396-7 44. Usenko D.V., Gorelov A.V., Samarin A.S., Shabalina S.V. Clinical and laboratory characteristics of viral intestinal infections in children with concomitant allergic pathology. Proceedings of IV Congress of Pediatricians of the CIS "Child and Society: health problems, development and nutrition ", April 25-26, 2012, Lviv, Ukraine. p.342 45. Usenko D.V., Gorelova E.A. State of the GIT microflora in acute intestinal infections in children with atopic dermatitis. Materials of III Interregional Scientific and Practical Conference "Infectious diseases in adults and children. Recent issues of diagnosis, treatment and prevention. " September 24-25, 2012, Astrahan.pp.148-50. 46. Kozhevnikova E.N., Usenko D.V., Nikolaeva S.V., Elezova L.I. Products with probiotics- an important component of functional foods. Pediatrics. Journal named after G.N. Speransky, 2012 Vol. 91. №4. pp. 72-78. * 47. Usenko D.V., Gorelov A.V., Shabalin S.V., Gorelova E.A. Clinical and laboratory features of acute intestinal infections in children with atopic dermatitis. Pediatrics. . Journal named after G.N. Speransky, 2013. Vol. 92. №1. pp. 40-45. * * - The magazines named in the list of State Commission for academic degrees and titles.