To examine the efficacy of Omalizumab in patients with Atopic
advertisement

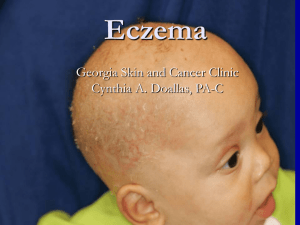
To examine the efficacy of Omalizumab in patients with Atopic Dermatitis Advisor:黃憲斌 Speaker:許寶寶 Date:2015.10.02 Atopic dermatitis is an inflammatory skin disorder which is increasing in incidence, and is characterized by severe pruritus, a chronically relapsing course, a distinctive distribution of eczematous skin lesions, and a personal or family history of atopic diseases. The pathogenesis of AD has been attributed to a complex interaction including environmental factors, host susceptibility genes, altered skin barrier function and a deregulated immune system. Many studies addressing the pathophysiology of AD have focused on processes potentially involving the regulation of IgE, which is higher than normal in 80% of all patients. Factors that influence IgE production include cytokines (like interleukins-4 and -13) and inflammatory cells that are present in the milieu. When antigen cross-links the specific IgE bound to the FceRI on mast cells and basophils, these cells initiate and amplify the inflammatory response in both the airways and atopic skin. Omalizumab is a chimeric monoclonal antibody approved for treating atopic patients with moderate to severe persistent allergic asthma with a serum IgE ranging from 30 to 700 IU/mL.Omalizumab binds IgE at the same binding site as FceRI and FceRII, thereby inhibiting mast cell or basophil activation. Some studies demonstrated a significant benefit from anti-IgE in the treatment of atopic dermatitis by decreasing serum IgE levels, and decreasing the using corticosteroid dose, and improvement in quality of life. So we hope our patients with atopic dermatits get improvement in cutaneous symptoms as well as laboratory results with using anti-IgE treatment. So we are going to examine the efficacy of omalizumab for the treatment of atopic dermatitis. A prospective analysis will perform to assess the efficacy of omalizumab in 10 patients with AD. Major inclusion criteria included patients with ages 12-65 years, diagnosis of AD, and showed IgE-mediated hypersensitivity to allergens. Major exclusion criteria included previous treatment with omalizumab, previous adverse reaction to omalizumab, pregnancy, other skin disease; psoriasis. Once enrolled, patients will be closely monitored at their routine visits. All patients will be observed for 60 minutes after injection. Assessment of clinical changes and any adverse effects will be conducted at every patient visit. These assessments include a full physical examination,vital signs, and a detailed patient history of adverse effects at each visit before their subsequent injection. An Investigator Global Assessment (IGA), based on a modified Scoring Atopic Dermatitis (SCORAD) index, will be used to calculate the severity of AD in each patient. These scores will be calculated at months 0, 1, 2, 3, 4, 5 and 6 after starting omalizumab.The SCORAD will be scored by the same investigator to prevent interobservator variability. Six markers of intensity — erythema or darkening, edema or papulation, oozing or crusting, excoriations, lichenification or prurigo, and dryness of uninvolved skin—will be assessed to define the severity of AD lesions. Each marker of intensity ranged from 0 to 3 points (0 none, 1 mild, 2 moderate, and 3 severe). A final modified IGA score ranged from 0 to 18 (1–6 mild AD, 7–12 moderate AD, 13–18 severe AD). Serum I-gE, total eosinophil count and cationic protein will be checked at month 0,1,2,3,4,5,6.