1: QUANTITATIVE CHEMISTRY OBJECTIVES
advertisement
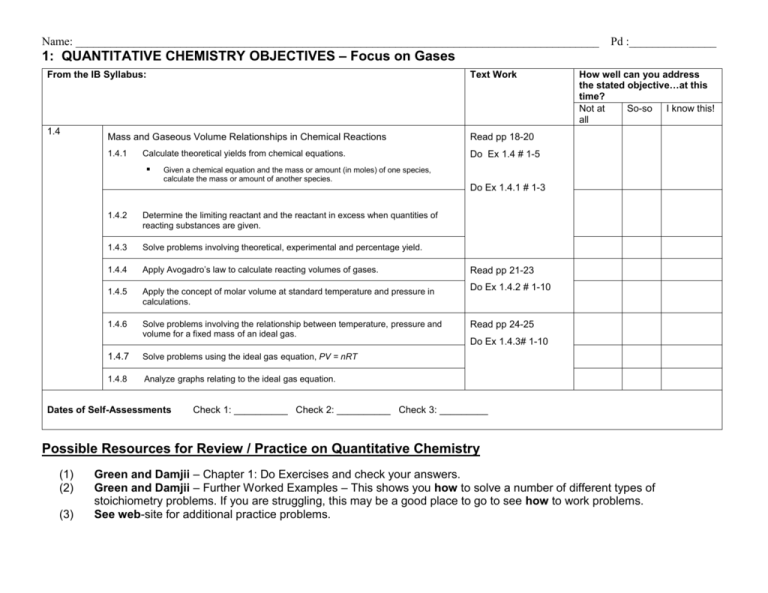
Name: __________________________________________________________________________________________ Pd :_______________ 1: QUANTITATIVE CHEMISTRY OBJECTIVES – Focus on Gases From the IB Syllabus: 1.4 Text Work Mass and Gaseous Volume Relationships in Chemical Reactions Read pp 18-20 1.4.1 Do Ex 1.4 # 1-5 Calculate theoretical yields from chemical equations. How well can you address the stated objective…at this time? Not at So-so I know this! all Given a chemical equation and the mass or amount (in moles) of one species, calculate the mass or amount of another species. Do Ex 1.4.1 # 1-3 1.4.2 Determine the limiting reactant and the reactant in excess when quantities of reacting substances are given. 1.4.3 Solve problems involving theoretical, experimental and percentage yield. 1.4.4 Apply Avogadro’s law to calculate reacting volumes of gases. 1.4.5 Apply the concept of molar volume at standard temperature and pressure in calculations. 1.4.6 Solve problems involving the relationship between temperature, pressure and volume for a fixed mass of an ideal gas. 1.4.7 Solve problems using the ideal gas equation, PV = nRT 1.4.8 Analyze graphs relating to the ideal gas equation. Dates of Self-Assessments Read pp 21-23 Do Ex 1.4.2 # 1-10 Read pp 24-25 Do Ex 1.4.3# 1-10 Check 1: __________ Check 2: __________ Check 3: _________ Possible Resources for Review / Practice on Quantitative Chemistry (1) (2) (3) Green and Damjii – Chapter 1: Do Exercises and check your answers. Green and Damjii – Further Worked Examples – This shows you how to solve a number of different types of stoichiometry problems. If you are struggling, this may be a good place to go to see how to work problems. See web-site for additional practice problems. Name: __________________________________________________________________________________________ Pd :_______________ 1: QUANTITATIVE CHEMISTRY OBJECTIVES - – Focus on Gases From the IB Syllabus: 1.4 Text Work Mass and Gaseous Volume Relationships in Chemical Reactions Read pp 18-20 1.4.8 Do Ex 1.4 # 1-5 Calculate theoretical yields from chemical equations. How well can you address the stated objective…at this time? Not at So-so I know this! all Given a chemical equation and the mass or amount (in moles) of one species, calculate the mass or amount of another species. Do Ex 1.4.1 # 1-3 1.4.9 Determine the limiting reactant and the reactant in excess when quantities of reacting substances are given. 1.4.10 Solve problems involving theoretical, experimental and percentage yield. 1.4.11 Apply Avogadro’s law to calculate reacting volumes of gases. 1.4.12 Apply the concept of molar volume at standard temperature and pressure in calculations. 1.4.13 Solve problems involving the relationship between temperature, pressure and volume for a fixed mass of an ideal gas. Read pp 21-23 Do Ex 1.4.2 # 1-10 Read pp 24-25 Do Ex 1.4.3# 1-10 1.4.14 Solve problems using the ideal gas equation, PV = nRT 1.4.8 Analyze graphs relating to the ideal gas equation. Dates of Self-Assessments Check 1: __________ Check 2: __________ Check 3: _________ Possible Resources for Review / Practice on Quantitative Chemistry (1) (2) (3) Green and Damjii – Chapter 1: Do Exercises and check your answers. Green and Damjii – Further Worked Examples – This shows you how to solve a number of different types of stoichiometry problems. If you are struggling, this may be a good place to go to see how to work problems. See web-site for additional practice problems.