A Model for Reaction Rates
advertisement

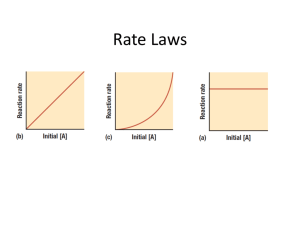
Average Rate = quantity time The amount of increase or decrease depends on mole ratios Units =Molarity/s or mol/Ls As a reaction proceeds, there is a decrease in concentration of reactants and an increase in the concentration of the products N2 + 3H2 2NH3 N2 and H2 decrease in concentration over time, while NH3 increases in concentration over time. Average reaction rate Concentration M2 - Concentration M1 t2 - t1 [Concentration] time Reaction rates must always be positive If you get a negative reaction rate, change the sign to positive 1. 2. 3. Reacting substances (atoms, ions, or molecules) must collide. Reacting substances must collide with the correct orientation Reacting substances must collide with sufficient energy to form the activated complex. The minimum amount of energy that reacting particles must have to form the activated complex and lead to a reaction Symbol: Ea Direct influence on the rate of a reaction inc time inc energy inc time inc energy F Given H2 + Cl2 Time (s) 0.00 5.00 [Concentration] time 2HCl [H2] (M) 0.060 0.025 [Cl2] (M) 0.070 0.035 [HCl] (M) 0.00 0.035 1. Calculate the average reaction rate expressed in moles H2 consumed per liter per second 0.025 – 0.060 = 0.007 M/s 5.00 – 0.00 2. Calculate the average reaction rate expressed in moles Cl2 consumed per liter per second 0.035 – 0.070 5.00 – 0.00 = 0.007 M/s An expression that relates rate of a reaction and reactant concentration Rate of reaction depends on reactant concentrations never includes products Given: 2A + 3B 2C ◦ In general, rate = k [A]x [B]y Symbol: k (lowercase) A constant specific and unique for every reaction If k is large, products form quickly If k is small, products form slowly Exponents in rate law (x and y) Determine how much the rate depends on the respective concentration(s) Can ONLY be determined by experiment Overall order of a reaction is the sum of all orders (x + y) 2A + 3B Skeleton Rate = k [A]x [B]y 2C Overall order of reaction (x + y) If x=1, 1st order in A If y=2, 2nd order in B Overall order 1 + 2 = 3rd order Write the skeleton rate law 2H2O(l) 2H2(g) + O2(g) rate = k [H2O]x Example 1: rate = k [A] 1st order ◦ If concentration of A doubles, rate doubles ◦ If concentration of A triples, rate triples ◦ If concentration of A is halved, rate halves **directly proportional Example 2: rate = k [A]2 2nd order ◦ If concentration of A doubles, rate quadruples ◦ If concentration of A triples, rate is 9 times as fast **exponentially proportional Example 3: rate = k [A]0 0th order ◦ Recall: anything raised to the 0th power = 1 ◦ If the concentration of A changes in any way NOTHING happens to the rate ◦ Rate is not dependent on concentration ◦ Rate = k