Service Protocol Reference Guide for the Complementary Package
advertisement

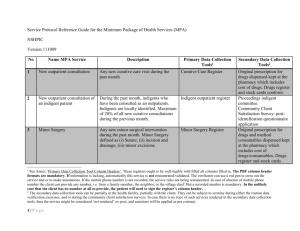
Service Protocol Reference Guide for the Complementary Package of Health Services (CPA) NSHPIC Version 111009 No Name CPA Service Description Primary Data Collection Tools1 1 New outpatient consultation by Any new curative care OPD visit attended a doctor by a Doctor during the evaluated month Curative Care Register 2 New outpatient consultation by Any new curative care OPD visit by an a doctor of an indigent patient indigent patient attended by a Doctor during the evaluated month. Indigents identified according to local norms. Maximum of 20% of all new curative consultations and or admissions during the previous month. Counter-referral slip arrived at A counter-referral note filled by the MD, the Health Center sent to the health center, during the evaluated month. The feedback must at Indigent register 3 Carbon copy of the original referral slip, filled in by the MD. Secondary Data Collection Tools2 Original prescription for drugs dispensed kept at the pharmacy which includes cost of drugs. Drugs register and stock cards conform. Lab/radiology register contains proof of requested exams. Proceedings Indigent committee Community Client Satisfaction Survey Original prescription for drugs and medical consumables dispensed kept See Annex ‘Primary Data Collection Tool Column Headers’. These registers ought to be well-legible with filled all columns filled in. The PBF column header formats are mandatory. If information is lacking, automatically this service is not remunerated/validated. The verificator can use a red pen to cross out the service and or to make annotations. If the mobile phone number is not recorded, the service risks not being remunerated. In case of absence of mobile phone number the client can provide any number, i.e. from a family member, the neighbor, or the village chief. But a recorded number is mandatory. In the unlikely case that the client has no number at all to provide, the patient will need to sign the register’s column header. 2 The secondary data collection tools can be partially at the health facility, partially with the client. They can be subject to scrutiny during either the routine data verification exercises, and or during the community client satisfaction surveys. In case there is no trace of such services rendered in the secondary data collection tools, then the service might be considered ‘not rendered’ ex-post, and sanctions will be applied as per contract. 1 1|Page No Name CPA Service Description Primary Data Collection Tools1 least mention the diagnosis and treatment received. The carbon copy of the referral note is only remunerated when it is accompanied by a short note with name, date and signature of the health center incharge. 4 Minor Surgery Any new minor surgical intervention during the evaluated month. Minor Surgery defined as (i) Suture; (ii) Herniotomy; (iii) Subcutaneous cyst removal; (iv) I&D; (v) amputation of a finger/toe Minor Surgery Register 5 Major Surgery (ex CS) Any new major surgical intervention during the evaluated month. Major surgical intervention defined as a laparatomy for any cause (bar CS), or amputation of a large limb. Theater register 6 Normal delivery A normal delivery attended by a trained attendant in this facility, during the Delivery register 2|Page Secondary Data Collection Tools2 at the pharmacy which includes cost of drugs/consumables. Drugs register and stock cards conform. Lab/radiology register contains proof of requested exams. Original referral slip available at the Health Center Original prescription for drugs and medical consumables dispensed kept at the pharmacy which includes cost of drugs/consumables. Drugs register and stock cards conform. Lab/radiology register contains proof of requested exams. Original prescription for drugs and medical consumables dispensed kept at the pharmacy which includes cost of drugs/consumables. Drugs register and stock cards conform. Lab/radiology register contains proof of requested exams. Partogram and inpatient file; eventual drugs and medical No Name CPA Service Description Primary Data Collection Tools1 evaluated month. 7 Assisted delivery An assisted delivery attended by a Doctor in this facility, during the evaluated month. Delivery register 8 CS A CS carried out at this facility during the evaluated month. Delivery register or theater register 9 Inpatient Day General admission register for each department 10 Postnatal consultation 11 First ANC consultation before four months pregnancy 12 ANC standard visit (2-4) One day admission of an admission of a minimum of three days duration and discharged alive, during the past month. A post natal consultation held within 48 hours after giving birth, during the past month. A first ANC consultation occurs before 4 month’s pregnancy, during the evaluated month. Any 2-4th standard visit according to the focused antenatal care visit schedule and approach. Second visit between 24-28 3|Page Secondary Data Collection Tools2 consumables dispensed through the prescriptions kept at the pharmacy; drugs register and stock cards conform. Partogram and inpatient file; eventual drugs and medical consumables dispensed through the prescriptions kept at the pharmacy; drugs register and stock cards conform. Partogram and inpatient file; eventual drugs and medical consumables dispensed through the prescriptions kept at the pharmacy; drugs register In patient form kept at the health facility ANC register ANC card kept at the health facility ANC register ANC card kept at the health facility. ANC register ANC card kept at the health facility. Medical prescriptions for No Name CPA Service Description Primary Data Collection Tools1 weeks; third visit at 32 weeks and the fourth visit at 36 weeks. During the evaluated month. 13 FP: total of new users of modern FP methods 14 FP: implants and IUDs 15 FP: vasectomy and bilateral tuba ligation 16 VCT/PMTCT/PIT test 17 PMTCT: HIV+ pregnant mothers and children born to are treated according to protocol STD treated 18 4|Page Any new or existing user of injectable contraceptive or oral contraceptive pills, during the past month. An injection represents three month’s protection and a FP visit for OAC should provide three month’s worth of pills. Any new user of implant or IUD, during the evaluated month. FP register A vasectomy and bilateral tuba ligation carried out at this facility, during the evaluated month Any new VCT or PMTCT or PIT test carried out during the evaluated month. Any new HIV+ mother and newborn child treated according to the PMTCT protocol, during the evaluated month. Theater register Any new STD treated according to the syndromic treatment protocol, during the evaluated month Curative Care Register FP register VCT/PMTC register ARV register; delivery room register Secondary Data Collection Tools2 Ferrosulphate, Mebendazole and Fansidar kept at the pharmacy. Drugs register and stock cards conform. Eventual drugs and medical consumables dispensed through the prescriptions kept at the pharmacy; drugs register and stock cards conform. Eventual drugs and medical consumables dispensed through the prescriptions kept at the pharmacy; drugs register and stock cards conform. Family Planning Register Laboratory register; stock records PMTCT register; laboratory register; stock records. Drugs and medical consumables dispensed through the prescriptions kept at the pharmacy; drugs register and stock cards No Name CPA Service 19 New Client put under ARV treatment 20 New AAFB+ PTB patient 21 PTB patient completed treatment and cured 5|Page Description Any new patient (pediatric or adult) HIV positive who started ARV (Antiretroviral therapy), including transferred in, during the evaluated month. A new AAFB sputum positive Pulmonary Tuberculosis patient diagnosed, at the facility, during the past month. A former AAFB+ PTB patient completed DOTS, and cured after treatment proven by negative sputum examinations, during the past month. Primary Data Collection Tools1 ART Register Tuberculosis register Tuberculosis register Secondary Data Collection Tools2 conform. Patient files Laboratory register: Slides kept for counterverification/quality assurance. Laboratory register: Slides kept for counterverification/quality assurance. Drugs register.