January 4 – Aromatic hydrocarbons
advertisement

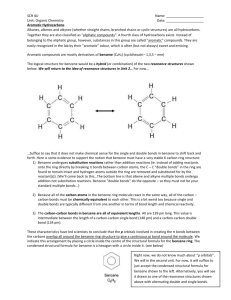
Chemistry 11 Aromatic Hydrocarbons - Notes 1. What is the difference between aliphatic hydrocarbons and aromatic hydrocarbons? 2. What is the molecular formula for benzene? 3. What is the structure of benzene? 4. Using words, describe a benzene molecule. 5. What are delocalized electrons? 6. Describe the single and double bonds of benzene. 7. Are these bonds stable? 8. Now draw the proper structure of benzene. 9. Illustrate what the prefixes ortho-, meta- and para- indicate. 10. Complete #32 – 35 page 361. Please hand these in. 11. Complete #3 (think “Like dissolves like”), 4, 5, 7 page 363 and 11a, 13 (not c) page 364. Chemistry 11 Aromatic Hydrocarbons - Notes 1. What is the difference between aliphatic hydrocarbons and aromatic hydrocarbons? 2. What is the molecular formula for benzene? 3. What is the structure of benzene? 4. Using words, describe a benzene molecule. 5. What are delocalized electrons? 6. Describe the single and double bonds of benzene. 7. Are these bonds stable? 8. Now draw the proper structure of benzene. 9. Illustrate what the prefixes ortho-, meta- and para- indicate. 10. Complete #32 – 35 page 361. Please hand these in. 11. Complete #3 (think “Like dissolves like”), 4, 5, 7 page 363 and 11a, 13 (not c) page 364. Chemistry 11 Aromatic Hydrocarbons - Notes 1. What is the difference between aliphatic hydrocarbons and aromatic hydrocarbons? 2. What is the molecular formula for benzene? 3. What is the structure of benzene? 4. Using words, describe a benzene molecule. 5. What are delocalized electrons? 6. Describe the single and double bonds of benzene. 7. Are these bonds stable? 8. Now draw the proper structure of benzene. 9. Illustrate what the prefixes ortho-, meta- and para- indicate. 10. Complete #32 – 35 page 361. Please hand these in. 11. Complete #3 (think “Like dissolves like”), 4, 5, 7 page 363 and 11a, 13 (not c) page 364. Chemistry 11 Aromatic Hydrocarbons - Notes 1. What is the difference between aliphatic hydrocarbons and aromatic hydrocarbons? 2. What is the molecular formula for benzene? 3. What is the structure of benzene? 4. Using words, describe a benzene molecule. 5. What are delocalized electrons? 6. Describe the single and double bonds of benzene. 7. Are these bonds stable? 8. Now draw the proper structure of benzene. 9. Illustrate what the prefixes ortho-, meta- and para- indicate. 10. Complete #32 – 35 page 361. Please hand these in. 11. Complete #3 (think “Like dissolves like”), 4, 5, 7 page 363 and 11a, 13 (not c) page 364.