Chemistry Exam review Answer Key Topics: Sample questions: Ch
advertisement

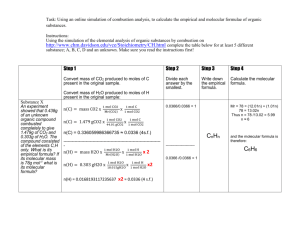
Chemistry Exam review Answer Key Topics: Sample questions: Ch 10--Pg 338 #59-60, 71-79, 88, 93, 97, 100; pg 343 #2, 3, 5, 8-14 o 59: find the moles of each atom from the formula, find the atomic mass of each element, multiply number of atoms by molar mass for each element and add together o 60 A. 98.0 g/mol, b. 76.0 g/mol, c. 100.1 g/mol, d. 132.1 g/mol, e. 89.0 g/mol o 71. 52.9% Al and 47.1% O o 72. 70% Fe and 30% O o 73. A. 5.9% H, 94.1% S; b. 22.6% N, 6.5% H, 19.4% C, 51.6% O; c. 41.7% Mg, 54.9% O, 3.4%H; d. 42.1% Na, 18.9% P, 39.0% O o 74. A. 3.33 g S, b. 5.65 g N, c. 40.6 g Mg, d. 152 g P o 75. D o 76. An empirical formula has the lowest whole-number ratio of elements; a. molecular, b. molecular, c. molecular and empirical, d. molecular and empirical o 77. A. molecular, b. molecular, c. empirical o 78. A o 79. A. C3H6O3, b. Hg2Cl2 o 88. A. CO, b. C2O2NH5, c. Cl2OC o 93. C3H6O3 o 97. A molecular formula is a whole-number multiple of its empirical formula o 100. A. C9H11O2N, b. C9H11O2N o 2. C o 3. A o 5. A o 8. E o 9. A o 10. D o 11. C o 12. C3H8O, 60.0 g/mol o 13. C2H5NO2, 75.0 g/mol o 14. C3H6O2, 74.0 g/mol Ch 11--Pg 377 #34, 36-37, 48-52, 54-55, 59; pg 381 #1, 3, 9-11 o 34. A. reacatants: sodium and water; products: hydrogen and sodium hydroxide; b. reactants: carbon dioxide and water; products: oxygen and glucose o 36. A formula is a unique identifier of a substance. A different formula would indicate a different substance, not the one that is taking part in the reaction you are trying to balance. o 37. A 2PbO2 (s) 2PbO (s) + O2 (g), b. 2Fe(OH)3 (s) Fe2O3 (s) + 3H2O (l), c. (NH4)2CO3 (s) 2 NH3 (g) + H2O (g) + CO2 (g), d. CaCl2 (aq) + H2SO4 (aq) CaSO4 (s) + 2HCl (aq) o 48. A. Cl2 (g) + 2 KI (aq) I2 (aq) + 2KCl (aq), b. 2 Fe(s) + 6HCl (aq) 2 FeCl3 (aq) + 3 H2 (g), c. P4O10 (s) + 6H2O (l) 4 H3PO4 (aq) o 49. A. Cl2 + 2NaI 2NaCl +I2, b. 2NH3 N2 +3H2, c. 4Na + O2 2Na2O o 50. A. ZnS + H2SO4 H2S + ZnSO4, b. NaOH + HNO3 H2O + NaNO3, c. 2KF + Ca(NO3)2 CaF2 + 2KNO3 o 51. A. Na2O + H2O 2NaOH, b. H2 + Br2 2HBr, c. Cl2O7 + H2O 2HClO4 o 52. A Fe + H2SO4 FeSO4 + H2, b. no reaction, c. Br2 + BaI2 BaBr2 + I2 o 54. A. 2C8H18 + 25 O2 16 CO2 + 18 H2O, b. C6H12O6 + 6O2 6CO2 + 6H2O, c. HC2H3O2 + 2O2 2 CO2 + 2 H2O o 55. A. 2 Al2O3 4Al + 3O2, b. Sn(OH)4 SnO2 + 2H2O, c. Ag2CO3 Ag2O + CO2 o 59. A. 3NaI + H3PO4 3HI + Na3PO4, double replacement, b. K2O + H2O 2 KOH, combination, c. 2H2SO4 2H2O + O2 + 2SO2, decomposition, d. 2Al + 3H2SO4 3H2 + Al2(SO4)3, single replacement, e. C5H12 + 8O2 5CO2 + 6H2O, combustion o 1. B o 3. C o 9. H2O, NH3, and CO2 o 10. (NH4)2CO3 2NH3 + CO2 + H2O o 11. Ca(OH)2 + CO2 CaCO3 + H2O 1st semester review questions (chapters 1-9): Pg R51: #10-13, 30-32, 36-39, 42-43, 47-49, 53, 55, 57-58, 60-66 o 10. 17.9 g o 11. 1.66 cm2 o 12. 13.1 kg o 13. A. 5.5 x 10 -2 g, b. 5.76 x 10 -1 L, c. 9.6 x 10 5 um, d. 5.26 x 10 -9 s, e. 8.7 x 10 7 mg, f. 8.46 x 10 1 cmol, g. 3.4 x 10 3 pm, h. 6.66 Mg, i. 2.34 x 10 -2 uL o 30. A. 18.0 g, b. $31.50 o 31. 52.8 cm3 sodium o 32. 4.95 x 103 kg o 36. A. not equal, b. equal, c. equal, d. not equal o 37. A. 23 protons, 23 electrons, 28 neutrons; b. 13 protons, 13 electrons, 14 neutrons; c. 50 protons, 50 electrons, 70 neutrons; 72 protons, 72 electrons, 106 neutrons o 38. A. molybdenum-98, b. helium-3, c. somium-189, d. gallium-71 o 39. 87.62 amu o 42. A. 1s22s22p63s23p64s23d8, b. 1s22s22p63s23p4, c. 1s22s22p63s23p64s23d104p3, d. 1s22s22p63s23p64s23d104p65s1 o 43. A. Cu, b. Cl, c. Zr, d. Kr o 47. A. Na, b. Cl, c. Br, d. Ne o 48. A. C, b. Al, c. Li, d. F o 49. A. F, b. F, c. Be, d. Ar o 53. A. 2, b. 3, c. 3, d. 2 o 55. MX2 and MX3 are valid formulas o 57. See other page with drawings o 58. A. 10, b. 26, c. 26, d. 18 o 60 a. polar, b. polar, c. nonpolar, d. nonpolar o 61. A. molecular, b, ionic, c, ionic, d, ionic, e. ionic, f. molecular, g. molecular, h. ionic o 62. A. silicon tetrachloride, b, PI3, c. dibronine heptoxide, d. IF, e. bromine pentafluoride, f. As2O3, g. nitrogen trichloride H. P2O5 o 63. A. BaI2, b. Fe(C2H3O2)3, c. K2Cr2O7, d. NH4Br, e. Cs3N, f. Co(NO3)3, g. Al2(C2O4)3, h. Hg2Cl2 o 64. A. rubidium sulfide, b. lithium iodide, c. lead(II) chloride, f. copper (I) chlorate, g. sodium cyanide, h. chromium (III) perchlorate o 65. A. cesium oxide, b. tin(II) sulfide, c. tetranitrogen tetrasulfide, d. diboron trioxide, e. chromic acid, f. calcium oxalate, g. ammonium phosphate, h. tetraarsenic decoxide o 66. A. CaO, b. H2SO3, c. B2Cl4, d. CaHPO4, e. SnCrO4, f. Fe(OH)3, g. Mn(ClO2)2, h. ICl What is the goal of distillation? Describe how this process works—include the physical properties that are used to separate the components. o Distillation separates a mixture based on boiling point. It is typically used to separate liquid mixtures. By heating the mixture, the substance with a lower boiling point will evaporate and boil away before the substances with higher boiling points. The vapor is then condensed back into a liquid into separate containers Devise a method by which sawdust, iron fillings, water and salt could be separated. Describe what you would expect to see after each item was separated. o Stir the mixture to dissolve the salt in the water. Allow to settle: iron fillings should sink, sawdust should form a orangish film at the top. Skim off the orange film and gently heat to dry. Carefully pour the liquid off the gray sediment, using a magnet to contain the sediment into one location. Dry the sediment and boil the liquid (distill) to separate the salt and water. Should end up with a light orange/yellow powder, a grey powder, a white powder or crystal and a clear liquid. Four coffee cups each contain the same volume of liquid. Two cups contain water and the other two contain rubbing alcohol. One cup of with each type of liquid is heated, which the other is cooled. You have a thermometer and a stopwatch to take measurements. Write the best hypothesis that can be tested using these materials and these conditions. o The type of substance or chemical composition determines how fast or how slowly the substance’s temperature will change. o Rubbing alcohol will change temperature faster (or slower) than water. (both options would be valid hypothesis) Using the image below, write down the measurements for both objects A and B for all three rulers. Which ruler was the most precise instrument? Object A: Ruler 1—6.79 cm, Ruler 2—6.6, Ruler 3—6.5 Object B: Ruler 1—4.48 cm, Ruler 2—4.3, Ruler 3—4.2 Ruler 1 is most precise Which set of data is more precise: 2.34 cm, 2.89 cm, and 2.12 cm or 5.96 cm, 5.89 cm, and 5.91 cm? If the actual value for the objects being measured are 2.45 cm and 5.92 cm respectively, which set has the lowest percent error? Which set is the most accurate? Why? o The second set is more precise and has the lowest percent error because the numbers in the set are closer together and all values are closer to the actual value in the second set. The second set is also the most accurate because one of the values in the second set is .01 away from the actual value, compared to the closest from set one which is .09 away from the actual. The graph shows the relationship between the mass of radioactive strontium-90 as a function of time. Estimate the half-life of strontium-90. Describe the process by which you can determine the half-life from this graph. The half life is 25 years, based on the fact that you started with 100 g, and when the sample was at 50 g, 25 years had elapsed. Alternatively, the loss of material between the 50 g and 25 g amount of strontium-90 corresponds to the change in time of 25 to 50 years—which would also indicate a half-life of 25 years. The green balloons represent the fluorine atoms bonded to a single sulfur atom (yellow in the center). What is the name of the compound this model represents? Sulfur hexafluoride