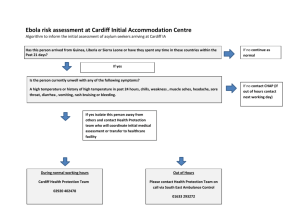
One Medicine and Genomic Pathology: How the Mouse Has

One Medicine: The Biological Significance of Mouse Models of Cancer.
Robert D. Cardiff, MD, PhD
Center for Comparative Medicine
Center of Genomic Pathology
University of California, Davis CA 95616 rdcardiff@ucdavis.edu http://ctrgenpath.org
Mouse fanciers have long known that mice get cancer. However, the issue is whether these tumors are really like our human cancers. Those who sought to learn from the study of mice have always been criticized. Regardless, mice teach have taught us lessons about the biology of cancer that could never been inferred from the human disease 1 .
The Genomic era has used mice for a modern version of Koch’s (Pasteur’s”) postulates 2 . The way to find whether a given gene causes a disease is to place it into a mouse. In cancer research, genetic engineering has confirmed the oncogenic potential of numerous genes, uncovered numerous genes that modify neoplastic progression and defined tumor suppressor genes by knocking out the suspect genes. Genetic engineering has revealed unsuspected relationships 3 .
The successes of Mouse Models of cancer initially came at the outset of genetic engineering with the ability to test gene function in any organ. The same oncogenic transgenes proved to cause cancers when expressed in different organs 3 . The silencing of tumor suppressor genes demonstrated common effects in different organs. Inducible genes led to the concepts of oncogene addiction and non-oncogene addiction 4 . The ability to study the effect of a single oncogene led to recognition of oncogene specific signature phenotypes pathology
5 and pathway
6 . The surprising recurrent tumors escaping oncogene addiction brought the
Epithelial-to-Mesenchymal Transition (EMT) tumors to the fore 7 . The GEM continue to teach us about stem cell biology and show surprising new tumor phenotypes 8 .
Even with the successes of mouse modeling, critics continue to point out the short comings of mouse models 9 . Mice are not humans and models are approximations. So, what is the value of mouse models? What is a model?
A model approximates one or more attributes of the human disease Obviously, a model cancer will not have all of the traits of a human cancer. Therefore, the model must be chosen that, in fact, recapitulates the particular traits the investigator wants to study. Given these reservations, one can choose or design the appropriate model.
The most valuable attribute of mouse modeling, in my view, is the biology that is mentioned only secondarily in most publications. The biology is informative and frequently frustrates the molecular biologists because of the major differences in the biology of mouse and man. Since the anatomy and physiology of mice are different from human, one expects, and observes, major differences in their tumor biology 10 . For example, very few mouse mammary, prostate
and colorectal tumors metastasize 11 . Most mouse tumors have expansile boarders rather than invasive and little of the fibrosis found in human tumors. The other side of these disappointments come with the observation of non-invasive intravascular metastasis in mouse and, subsequently, in human cancers and genes that control the metastatic phenotype 12 . The
Test-by-Transplantation, that is only available in mice, has provided operational definitions of pre-cancer, cancer and metastasis permitting studies of neoplastic progression that can only be
inferred in humans 13,14 This test has been used to prove that the hypothesis of
“one step induction” was false 15
and that the TRAMP cancers are neuroendocrine 11,16,17 . Gem models are
being used to trace lineages opening new avenues for lineage and stem cell research 18 .
The development of “Mouse Hospitals” for the conduct of “co-clinical-” or “pre-clinical-“ trials using GEMM and PDX models has introduced a new level of complexity to modeling with the production of genomic modified mouse models “on-demand” from cryopreserved blastocystic stocks 19 .
The pathology community is now being asked to provide standard operating procedures for verification, validation, classification and certification of mouse models of human cancer. This call is in response to the large number of models and diverse published claims and the Mouse
Hospital movement.
Verification , in this context, simply implies that the process is identified. It sounds simple but how many of us have seen granulomas mistaken for tumors, or common BAC pulmonary tumors mistaken for metastasis 11 or remember the famous preputial glands that were mistaken for symmetrical “teratomas” 11 . Verification requires competent comparative pathologist familiar with mouse and man.
Validation , when applied to mouse models, is intended to mean that the attribute under study is correctly identified and reproducible. In many cases, uninformed investigators will mis-identify a process or apply an inappropriate term to the process 20 . A recent example was the application of the term “metastatic” to a process that was clearly direct extension from the primary 11
Classification requires a broad experience in the field that is seldom afforded to an isolated local institutional pathologist. However, in the modern world with digital whole slide images, remoteness can be overcome. Classification also requires a command of terminology with controlled vocabularies 21 .
Certification imagines the development of some type of objective scoring system that will identify the “best” mouse model for studying a particular set of attributes. The “certification” of models will require a new level of morphological and genomic scrutiny that will require an understanding and integration of diverse data sets. However, what the community needs is a record that lists the attributes of each model system that can guide its choice of models.
Standard Operating Procedures (SOP) are now required to bring some standardization to research. Those who have worked in a clinical setting or industry are quite familiar with SOP.
However, the basic science community has never had the discipline of SOP and operate laboratories with little or no restraints. What they need from pathology is some rudimentary directions such as illustrated in these chapters One Medicine: The biological Significance of
Mouse Models of Cancer 19,21-25 .
These are tasks that can only be accomplished by pathologists who can phenotype mice, understand their biology and accurately interpret gross and microscopic changes. This is your future. You have my best wishes.
CITATIONS
1.
2.
3.
4.
Cardiff RD, Kenney N. Mouse mammary tumor biology: a short history. Advances in cancer
research. 2007;98:53-116.
Cardiff RD, Kenney N. A compendium of the mouse mammary tumor biologist: from the initial observations in the house mouse to the development of genetically engineered mice. Cold
Spring Harb Perspect Biol. 2011;3(6).
Cardiff RD, Munn RJ, Galvez JJ. The Tumor Pathology of Genetically Engineered Mice: A New
Approach to Molecular Pathology. In: Fox JG, Davisson MT, Quimby FW, Barthold SW,
Newcomer CE, Smith AL, eds. The Mouse in Biomedical Research: Experimental Biology and
Oncology. . Vol 2. Second ed. New York: Elsevier, Inc; 2006:581-622.
Couto SS, Bolon B, Cardiff RD. Morphologic manifestations of gene-specific molecular alterations
("genetic addictions") in mouse models of disease. Vet Pathol. Jan 2012;49(1):116-129.
5.
6.
7.
Cardiff RD, Sinn E, Muller W, Leder P. Transgenic oncogene mice. Tumor phenotype predicts genotype. The American journal of pathology. Sep 1991;139(3):495-501.
Rosner A, Miyoshi K, Landesman-Bollag E, et al. Pathway pathology: histological differences between ErbB/Ras and Wnt pathway transgenic mammary tumors. The American journal of
pathology. Sep 2002;161(3):1087-1097.
Cardiff RD. The pathology of EMT in mouse mammary tumorigenesis. J Mammary Gland Biol
Neoplasia. Jun 2010;15(2):225-233.
8. Rudmann D, Cardiff R, Chouinard L, et al. Proliferative and nonproliferative lesions of the rat and mouse mammary, Zymbal's, preputial, and clitoral glands. Toxicol Pathol. Aug 2012;40(6
Suppl):7S-39S.
Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human 9. inflammatory diseases. Proc Natl Acad Sci U S A. Feb 26 2013;110(9):3507-3512.
10. Cardiff RD, Allison KH. Mammary Gland. In: Trueting PM, Dintzis SM, eds. Comparative Anatomy
and Histology: A Mouse and Human Atlas Amsterdam, San Diego: Elsevier; 2011:39-50.
11. Ittmann M, Huang J, Radaelli E, et al. Animal Models of Human Prostate Cancer: The Consensus
Report of the New York Meeting of the Mouse Models of Human Cancers Consortium Prostate
Pathology Committee. Cancer Res. May 1 2013;73(9):2718-2736.
12. Oshima RG, Lesperance J, Munoz V, et al. Angiogenic Acceleration of Neu Induced Mammary
Tumor Progression and Metastasis. Cancer Res. Jan 1 2004;64(1):169-179.
13. Cardiff RD, Borowsky AD. Precancer: sequentially acquired or predetermined? In: Fitzgerald RC, ed. Pre-invasive disease : pathogenesis and clinical management. New York Springer; 2011.
14. Cardiff RD, Borowsky AD. Precancer: sequentially acquired or predetermined? Toxicol Pathol.
2010;38(1):171-179.
15. Maglione JE, Moghanaki D, Young LJ, et al. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61(22):8298-8305.
16. Chiaverotti T, Couto SS, Donjacour A, et al. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol. Jan 2008;172(1):236-246.
17. Couto SS, Cardiff RD. The Genomic Revolution and Endocrine Pathology. Endocr Pathol. Aug 31
2008;19(3):139-147.
18. Wang ZA, Mitrofanova A, Bergren SK, et al. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat
Cell Biol. Mar 2013;15(3):274-283.
19. Cardiff RD, Miller CH, Munn RJ. Analysis of mouse model pathology: a primer for studying the anatomic pathology of genetically engineered mice. Cold Spring Harb Protoc. Jun
2014;2014(6):561-580.
20. Cardiff RD, Rosner A, Hogarth MA, Galvez JJ, Borowsky AD, Gregg JP. Validation: the new challenge for pathology. Toxicol Pathol. Mar-Apr 2004;32 Suppl 1:31-39.
21. Cardiff RD, Miller CH, Munn RJ, Galvez JJ. Structured reporting in anatomic pathology for coclinical trials: the caELMIR model. Cold Spring Harb Protoc. Jan 2014;2014(1):32-43.
22. Cardiff RD, Miller CH, Munn RJ. Manual immunohistochemistry staining of mouse tissues using the avidin-biotin complex (ABC) technique. Cold Spring Harb Protoc. Jun 2014;2014(6):659-662.
23. Cardiff RD, Miller CH, Munn RJ. Manual hematoxylin and eosin staining of mouse tissue sections.
Cold Spring Harb Protoc. Jun 2014;2014(6):655-658.
24. Cardiff RD, Miller CH, Munn RJ. Mouse tissue fixation. Cold Spring Harb Protoc. May
2014;2014(5).
25. Cardiff RD, Miller CH, Munn RJ. Limited mouse necropsy. Cold Spring Harb Protoc. May
2014;2014(5).