Classifying Matter: Pure Substances & Mixtures Worksheet
advertisement
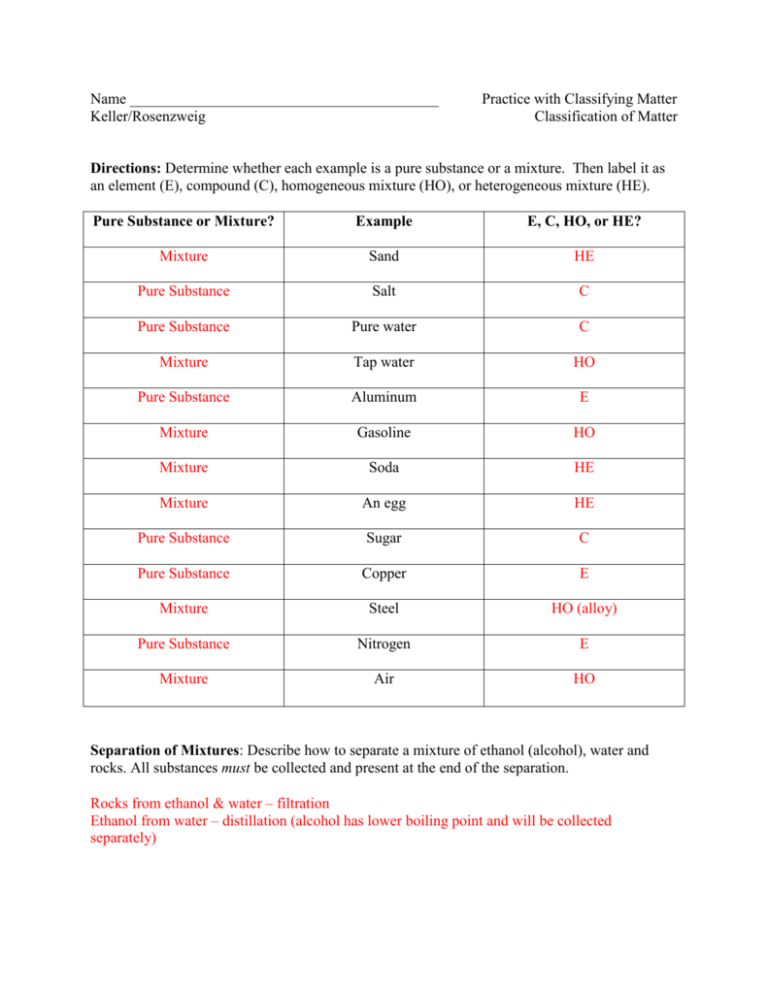
Name _________________________________________ Keller/Rosenzweig Practice with Classifying Matter Classification of Matter Directions: Determine whether each example is a pure substance or a mixture. Then label it as an element (E), compound (C), homogeneous mixture (HO), or heterogeneous mixture (HE). Pure Substance or Mixture? Example E, C, HO, or HE? Mixture Sand HE Pure Substance Salt C Pure Substance Pure water C Mixture Tap water HO Pure Substance Aluminum E Mixture Gasoline HO Mixture Soda HE Mixture An egg HE Pure Substance Sugar C Pure Substance Copper E Mixture Steel HO (alloy) Pure Substance Nitrogen E Mixture Air HO Separation of Mixtures: Describe how to separate a mixture of ethanol (alcohol), water and rocks. All substances must be collected and present at the end of the separation. Rocks from ethanol & water – filtration Ethanol from water – distillation (alcohol has lower boiling point and will be collected separately)