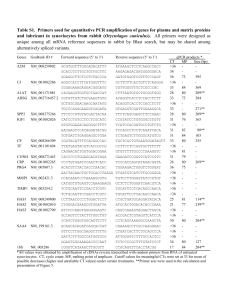
Supplementary Table 1 | The biochemical, biological and
advertisement

Supplementary Table 1 | The biochemical, biological and pharmacological properties of tivantinib are distinct to other MET inhibitors. Crizotinib, PHA-665752, JNJ-38877605, DN-30 Fab Selected based on their ability to inhibit MET tyrosine kinase activity or to cause MET downregulation Tivantinib Identified in a cell-based screen for pro-apoptotic agents Display selective activity in MET-addicted cells (displaying c-MET gene amplification) Displays cytotoxic activity towards all cells regardless of c-MET gene copy number or MET protein expression, including cells expressing kinase-dead MET or not expressing MET at all Fail to inhibit the growth of cells that have become Displays equal cytotoxicity towards MET inhibitorresistant to MET inhibition by multiple mechanisms sensitive and MET inhibitor-resistant cells Potently inhibit both HGF-dependent and HGFFails to inhibit either HGF-dependent or HGFindependent MET auto-phosphorylation and block independent MET auto-phosphorylation and does not downstream ERK-AKT signalling block downstream AKT/ERK signalling Cause G1 arrest of cells displaying c-MET gene Causes G2/M arrest and apoptosis of all cells amplification independently of c-MET genetic status and MET protein expression Have no effect on microtubule stability Interferes with microtubule polymerization independently of the presence or absence of a functional MET protein Crizotinib is a dual ALK-MET small-molecule inhibitor approved for non-small-cell lung cancer. PHA-665752 is a MET-selective small-molecule inhibitor that blocks HGF/MET-dependent biological activity in preclinical models. JNJ-38877605 is a MET-selective small-molecule inhibitor discontinued from clinical development owing to offtarget toxic effects. DN-30 Fab is the Fab fragment of a monoclonal anti-MET antibody inducing receptor shedding and downregulation.1,2 1 Figure 1 | The MET inhibitor herd. Basilico et al.1 and Katayama et al.2 directly compared the pharmacological properties of tivantinib with those of other MET inhibitors including crizotinib (a dual ALK-MET tyrosine kinase inhibitor (TKI) approved for non-small-cell lung cancer), PHA-665,752 (a preclinical MET TKI), JNJ-38877605 (a MET TKI inhibitor discontinued from clinical development owing to off-target toxic effects), and DN-30 (a monoclonal anti-MET antibody inducing receptor shedding and downregulation). Additional MET inhibitors not analysed in these studies include onartuzumab (a one-armed monoclonal anti-MET antibody in phase III for nonsmall-cell lung cancer and gastric cancer) and a number of MET-selective TKIs (AMG-337, EMD-1214063, EMD-1204831, SGX-523 and INCB28060) that are being tested in phase I in solid tumours. Additionally, antibodies have been developed to target HGF, the high affinity ligand for MET, including rilotumumab (in phase III for gastric cancer), ficlatuzumab (in phase II for non-small-cell lung cancer), and TAK-701 (in phase I in solid tumours). 2