Abstract - Department of Chemistry
advertisement
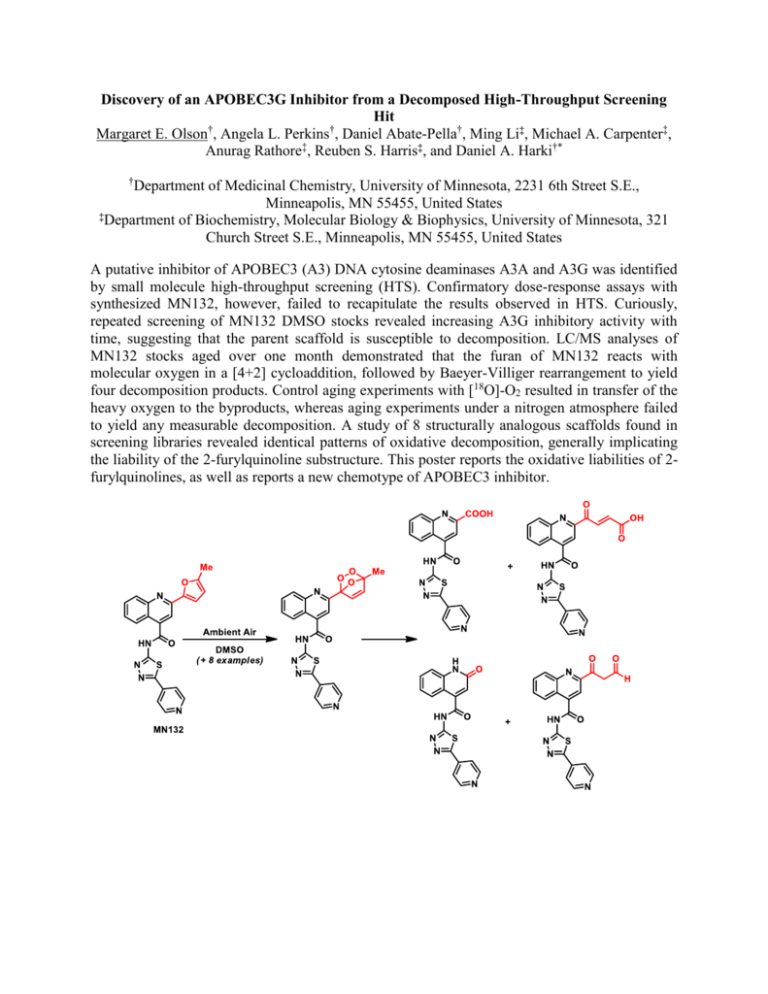
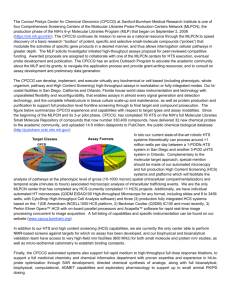
Discovery of an APOBEC3G Inhibitor from a Decomposed High-Throughput Screening Hit Margaret E. Olson†, Angela L. Perkins†, Daniel Abate-Pella†, Ming Li‡, Michael A. Carpenter‡, Anurag Rathore‡, Reuben S. Harris‡, and Daniel A. Harki†* † Department of Medicinal Chemistry, University of Minnesota, 2231 6th Street S.E., Minneapolis, MN 55455, United States ‡Department of Biochemistry, Molecular Biology & Biophysics, University of Minnesota, 321 Church Street S.E., Minneapolis, MN 55455, United States A putative inhibitor of APOBEC3 (A3) DNA cytosine deaminases A3A and A3G was identified by small molecule high-throughput screening (HTS). Confirmatory dose-response assays with synthesized MN132, however, failed to recapitulate the results observed in HTS. Curiously, repeated screening of MN132 DMSO stocks revealed increasing A3G inhibitory activity with time, suggesting that the parent scaffold is susceptible to decomposition. LC/MS analyses of MN132 stocks aged over one month demonstrated that the furan of MN132 reacts with molecular oxygen in a [4+2] cycloaddition, followed by Baeyer-Villiger rearrangement to yield four decomposition products. Control aging experiments with [18O]-O2 resulted in transfer of the heavy oxygen to the byproducts, whereas aging experiments under a nitrogen atmosphere failed to yield any measurable decomposition. A study of 8 structurally analogous scaffolds found in screening libraries revealed identical patterns of oxidative decomposition, generally implicating the liability of the 2-furylquinoline substructure. This poster reports the oxidative liabilities of 2furylquinolines, as well as reports a new chemotype of APOBEC3 inhibitor.