gcb12549-sup-0001-FigS1-S3
advertisement
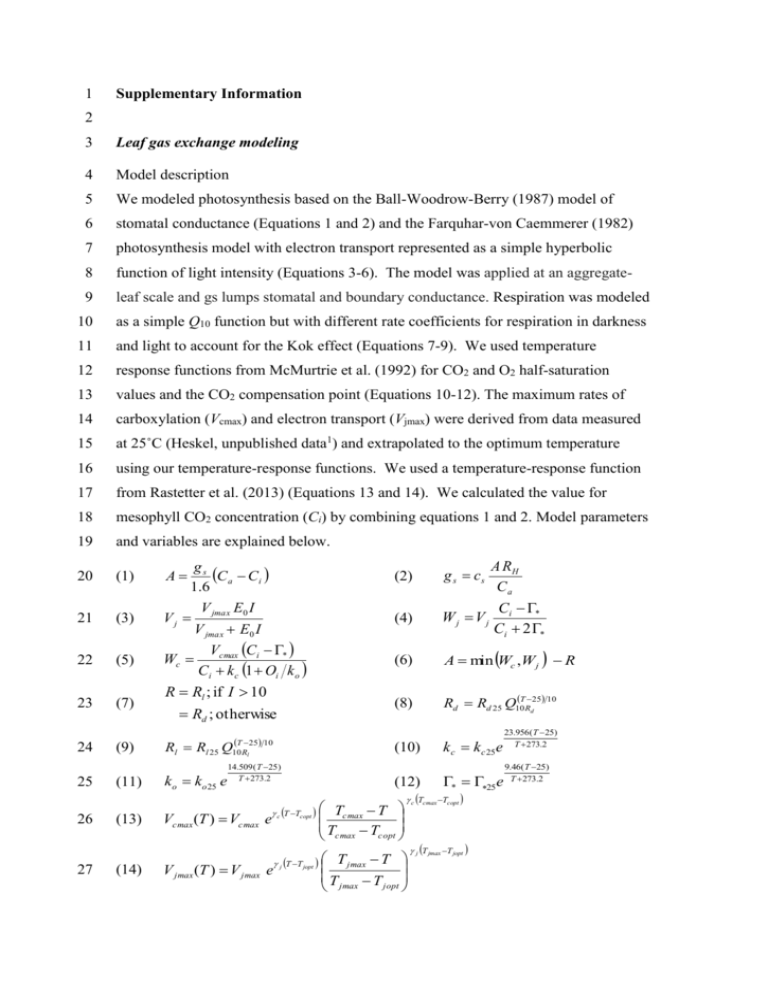
1 Supplementary Information 2 3 Leaf gas exchange modeling 4 Model description 5 We modeled photosynthesis based on the Ball-Woodrow-Berry (1987) model of 6 stomatal conductance (Equations 1 and 2) and the Farquhar-von Caemmerer (1982) 7 photosynthesis model with electron transport represented as a simple hyperbolic 8 function of light intensity (Equations 3-6). The model was applied at an aggregate- 9 leaf scale and gs lumps stomatal and boundary conductance. Respiration was modeled 10 as a simple Q10 function but with different rate coefficients for respiration in darkness 11 and light to account for the Kok effect (Equations 7-9). We used temperature 12 response functions from McMurtrie et al. (1992) for CO2 and O2 half-saturation 13 values and the CO2 compensation point (Equations 10-12). The maximum rates of 14 carboxylation (Vcmax) and electron transport (Vjmax) were derived from data measured 15 at 25˚C (Heskel, unpublished data1) and extrapolated to the optimum temperature 16 using our temperature-response functions. We used a temperature-response function 17 from Rastetter et al. (2013) (Equations 13 and 14). We calculated the value for 18 mesophyll CO2 concentration (Ci) by combining equations 1 and 2. Model parameters 19 and variables are explained below. 20 (1) 21 (3) 22 (5) 23 (7) 24 25 26 27 (9) (11) (13) (14) gs C a Ci 1 .6 V jmax E0 I Vj V jmax E0 I A Wc Vcmax Ci * Ci k c 1 Oi k o R Rl ; if I 10 Rd ; otherwise T 25 10 Rl Rl 25 Q10 Rl ko ko 25 e 14.509 ( T 25 ) T 273.2 Vcmax (T ) Vcmax e c T Tcopt V jmax (T ) V jmax e j T T jopt (2) g s cs A RH Ca (4) Wj Vj Ci * Ci 2* (6) A min Wc ,W j R (8) Rd Rd 25 Q10TRd25 10 (10) k c k c 25e (12) * 25e Tcmax T T cmax Tcopt c Tcmax Tcopt T jmax T T T jopt jmax j T jmax T jopt 23.956( T 25) T 273.2 9.46 ( T 25) T 273.2 Parameters and variables Abbreviation Unit Value Source Parameters Maximum carboxylation rate CO2 1/2 saturation point at 25 ˚C O2 1/2 saturation point at 25 ˚C Maximum electron transport rate Quantum yield CO2 compensation point at 25 ˚C Ball-Berry coefficient dark respiration at 25 ˚C Q10 for dark respiration light respiration at 25 oC Q10 for light respiration Maximum temperature of carboxylation Optimum temperature of carboxylation Temp sensitivity of carboxylation Maximum temperature of electron transport Optimum temperature of electron transport Temp sensitivity of electron transport Atmospheric CO2 concentration Intracellular O2 concentration Vcmax kc25 ko25 Vjmax E0 Γ*25 cs Rd25 Q10Rd Rl25 Q10Rl Tcmax Tcopt γc Tjmax Tjopt γj Ca Oi μmol m-2 leaf area s-1 μmol mol-1 μmol mol-1 μmol m-2 leaf area s-1 mol mol-1 μmol mol-1 None μmol m-2 leaf area s-1 None μmol m-2 leaf area s-1 None ˚C ˚C ˚C-1 ˚C ˚C ˚C-1 μmol mol-1 μmol mol-1 Various1 310 155 Various1 0.07 42 0.3 Various* Various* Various* Various* 61.171 38.595 0.126 55.171 32.852 0.168 390 200 Heskel et al., unpublished McMurtrie et al. (1992) McMurtrie et al. (1992) Heskel et al., unpublished Shaver et al. (2007) McMurtrie et al. (1992) Ball et al. (1987) This study This study This study This study Rastetter et al. (2013) Rastetter et al. (2013) Rastetter et al. (2013) Rastetter et al. (2013) Rastetter et al. (2013) Rastetter et al. (2013) A R gs RH I Ci Wc Vj μmol m-2 leaf area s-1 μmol m-2 leaf area s-1 mol m-2 leaf area s-1 None μmol m-2 s-1 μmol mol-1 μmol m-2 leaf area s-1 μmol m-2 leaf area s-1 Wj μmol m-2 leaf area s-1 Calculated variables CO2 assimilation rate Respiration rate Stomatal conductance Relative humidity Irradiance Mesophyll intracellular CO2 concentration Rubisco-limited rate of photosynthesis Rate of electron transport Light-limited rate of photosynthesis allowed by RuBP regeneration 1 2 Heskel, M. et al., unpublished data Species B. nana E. vaginatum 3 4 5 6 7 8 9 * Treatment GH CT GH CT Vcmax 25°C 42.26 43.76 38.95 28.42 depends on species and treatment (reported in this study) Vjmax 25°C 57.71 61.26 48.32 40.07 1 Measurements of leaf carbon and nitrogen characteristics 2 Methods 3 To interpret the potential influence of conditions in the greenhouses, we measured 4 leaf C and Nitrogen (N) characteristics. We collected leaf samples of E. vaginatum 5 from each replicate plant in each block. Samples were dried overnight at 60 degrees 6 ˚C, and ground to a fine powder. Approximately 4 mg of sample was used for analysis 7 of δ15N and δ13C isotopes using a NC2500 elemental analyzer (CE Instruments, 8 Wigan, UK) interfaced to a Thermo Finningan Delta Plus XP isotope ratio mass 9 spectrometer (IRMS) (Thermal Scientific, Waltham, MA, USA). Isotope ratios were 10 expressed relative to PeeDee Belemnite for δ13C and Air for δ15N. For E. vaginatum, 11 we compared C/N ratios, δ13C and δ15N values in the greenhouse and control 12 treatments with ANOVA that accounted for the block effect. 13 14 Results 15 We found no difference in δ13C values between treatments in E. vaginatum (Figure 16 4a). However, we found lower nitrogen concentration (ANOVA, p = 0.04), and higher 17 C/N ratios (ANOVA, p=0.003) in the greenhouse than in the control (SI Figure 3b, c, 18 d). 19 20 21 22 23 24 25 26 27 1 References 2 Ball, JT, IE Woodrow, and JA Berry. 1987. A model predicting stomatal conductance 3 and is contribution to the control of photosynthesis under different 4 environmental conditions. pp 221-224 in J Biggins (Ed.) Progress in 5 photosynthesis research. Martinus Nijhoff Publishers, The Netherlands 6 7 Farquhar, G.D., von Caemmerer, S., 1982. Modeling of photosynthetic responses to 8 environmental conditions. pp. 549–587 in Lange, O.L., Nobel, P.S., Osmond, 9 C.B., Ziegler, H. (Eds.), Encyclopedia of Plant Physiology (New Series). 10 Springer-Verlag, Berlin. 11 12 McMurtrie, R.E., H.N .Comins, M.U.F. Kirschbaum and Y.P. Wang 1992. Modifying 13 existing forest growth models to take account of effects of elevated CO2. 14 Australian Journal of Botany 40:657–677. 15 16 Rastetter, EB, R.D. Yanai, R.Q. Thomas, M.A. Vadeboncoeur, T.J. Fahey, M.C. Fisk, B.L. 17 Kwiatkowski, and S.P. Hamburg. 2013. Recovery from disturbance requires 18 resynchronization of ecosystem nutrient cycles. Ecological Applications 23:621-642. 19 20 Shaver, GR, LE Street, EB Rastetter, MT van Wijk, and M Williams. 2007. 21 Functional convergence in regulation of net CO2 flux in heterogeneous tundra 22 landscapes in Alaska and Sweden. Journal of Ecology 95:802-817 23 1 2 3 SI Figure 1. Representative light-response (AQ) curve. The Kok method measures the 4 response of A over incrementally decreasing irradiance. Gray circles represent the 5 points of the light response that lie above the break in the slope, which we extrapolate 6 back to RL on the y-axis. Black triangles represent the points on the light response 7 curve below the break in the slope and extend to RD on the y-axis at 0 PAR. 8 % Inhibition of RL 60 50 Control Greenhouse 40 30 20 10 a -2 -1 Vo (mol m s ) 0 1.2 1.0 0.8 0.6 0.4 0.2 b 0.0 1 E. vaginatum B. nana 2 SI Figure 2. Leaf respiratory inhibition (a) and Vo at 100 μmol PPFD m-2 s-1 (b) of E. 3 vaginatum and B. nana in control and greenhouse treatments. 4 5 0 5 ** -5 4 3 -15 15N 13C -10 2 -20 1 -25 aa b b -30 3.0 * ** 25 2.0 20 1.5 15 1.0 10 0.5 5 c d 0.0 0 Control Greenhouse Control Greenhouse 1 2 SI Figure 3. Leaf (a) δ13C values, (b) δ15N values, (c) %N and (d) C/N ratios of E. 3 vaginatum in greenhouse and control treatments. Significant differences between the 4 control and greenhouse treatment are marked (* P<0.05, ** P<0.01). 5 C:N N% 2.5 0 30











