Section 3.1 Answers
advertisement

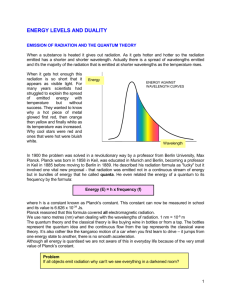
EARLY ATOMIC THEORIES AND THE ORIGINS OF QUANTUM THEORY Section 3.1 Answers 1. Electron/scanning 2. Electron charge 3. D 4. a) 6 electrons, 7 neutrons b) 7 e, 7n c) 19 e, 22 n d) 30 e, 36 n 5. atomic number is same but atomic masses are different 6. a) 41K19 b) 14N7 c) 59Co27 d) 102Ru44 7 a) 63Cu2+29 b) 32S2-16 9. Alpha, beta and gamma 10. c Plancks Theory 11. In 1900 Max Planck proposed a formula. Instead of allowing energy to be continuously distributed among all frequencies, Planck's model required that the energy in the atomic vibrations of frequency f was some integer times a small, minimum, discrete energy, Emin = hf where h is a constant, now known as Planck's constant, h = 6.626176 x 10-34 J s Planck's proposal, then requires that all the energy in the atomic vibrations with frequency f can be written as E=nhf where n in an integer, n = 1, 2, 3, . . . No other values of the energy were allowed. This idea that something -- the energy in this case -- can have only certain discrete values is called quantization. Photon Energies for EM Spectrum Answer 12. In 1905 Albert Einstein provided a daring extension of Planck's quantum hypothesis and was able to explain the photoelectric effect in detail. Expanding on Planck's quantum idea, Einstein proposed that the energy in the light was not spread uniformly throughout the beam of light. Rather, the energy of the light is contained in "packets" or quanta (the plural of quantum, a single "packet") each with energy of E=hf where again h is Planck's constant and f is the frequency of the light. All of the energy in one quantum -- now called a photon -- is given to one electron. Planck’s Law Every physical body spontaneously and continuously emits electromagnetic radiation. Near thermodynamic equilibrium, the emitted radiation is nearly described by Planck's law. Because of its dependence on temperature, Planck radiation is said to be thermal. The higher the temperature of a body the more radiation it emits at every wavelength. Planck radiation has a maximum intensity at a specific wavelength that depends on the temperature. For example, at room temperature (~300 K), a body emits thermal radiation that is mostly infrared and invisible. At higher temperatures the amount of infrared radiation increases and can be felt as heat, and the body glows visibly red. At even higher temperatures, a body is dazzlingly bright yellow or blue-white and emits significant amounts of short wavelength radiation, including ultraviolet and even x-rays. The surface of the sun (~6000 K) emits large amounts of both infrared and ultraviolet radiation; its emission is peaked in the visible spectrum. In the interior of a physical medium, radiation can be absorbed and emitted by matter. This mediates transfer of energy as heat, and can change the internal energy of the matter, and the occupation numbers of the states of its molecules. Planck radiation is the greatest amount of radiation that any body at thermal equilibrium can emit from its surface, whatever its chemical composition or surface structure. Passage of radiation across an interface between media can be characterized by the emissivity of the interface, the radiance of the passing radiation divided by the Planck radiance. It is in general dependent on chemical composition and physical structure, on temperature, on the wavelength, on the angle of passage, and on the polarization, of the radiation