zvalaydon_372_20130610233237
advertisement
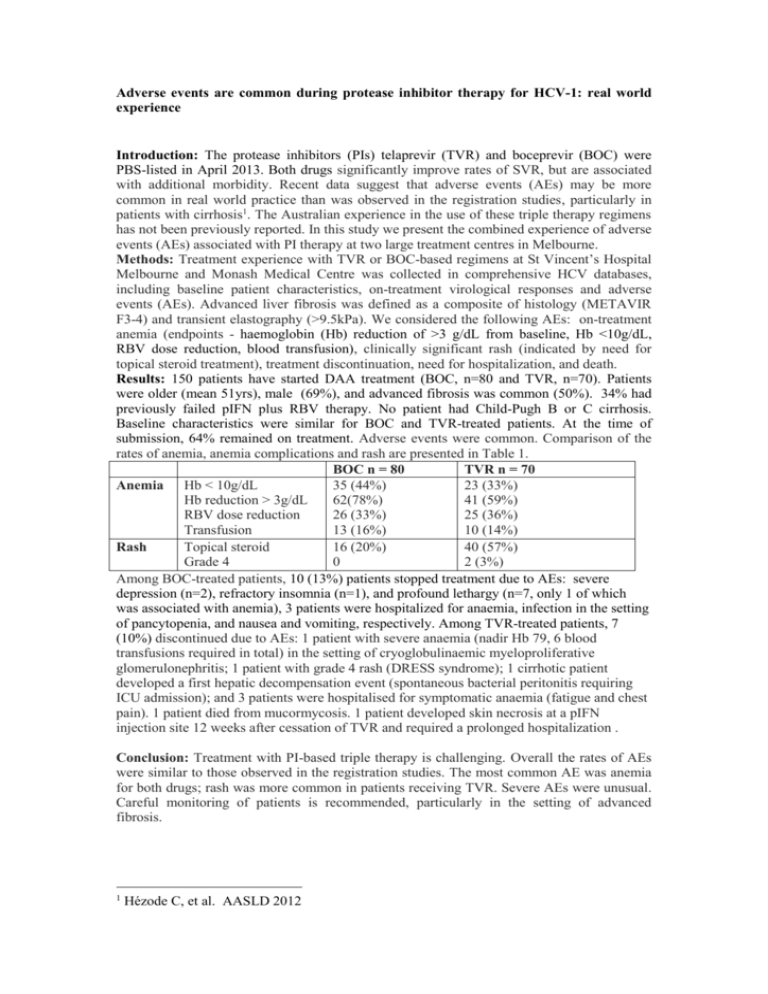
Adverse events are common during protease inhibitor therapy for HCV-1: real world experience Introduction: The protease inhibitors (PIs) telaprevir (TVR) and boceprevir (BOC) were PBS-listed in April 2013. Both drugs significantly improve rates of SVR, but are associated with additional morbidity. Recent data suggest that adverse events (AEs) may be more common in real world practice than was observed in the registration studies, particularly in patients with cirrhosis1. The Australian experience in the use of these triple therapy regimens has not been previously reported. In this study we present the combined experience of adverse events (AEs) associated with PI therapy at two large treatment centres in Melbourne. Methods: Treatment experience with TVR or BOC-based regimens at St Vincent’s Hospital Melbourne and Monash Medical Centre was collected in comprehensive HCV databases, including baseline patient characteristics, on-treatment virological responses and adverse events (AEs). Advanced liver fibrosis was defined as a composite of histology (METAVIR F3-4) and transient elastography (>9.5kPa). We considered the following AEs: on-treatment anemia (endpoints - haemoglobin (Hb) reduction of >3 g/dL from baseline, Hb <10g/dL, RBV dose reduction, blood transfusion), clinically significant rash (indicated by need for topical steroid treatment), treatment discontinuation, need for hospitalization, and death. Results: 150 patients have started DAA treatment (BOC, n=80 and TVR, n=70). Patients were older (mean 51yrs), male (69%), and advanced fibrosis was common (50%). 34% had previously failed pIFN plus RBV therapy. No patient had Child-Pugh B or C cirrhosis. Baseline characteristics were similar for BOC and TVR-treated patients. At the time of submission, 64% remained on treatment. Adverse events were common. Comparison of the rates of anemia, anemia complications and rash are presented in Table 1. BOC n = 80 TVR n = 70 35 (44%) 23 (33%) Anemia Hb < 10g/dL Hb reduction > 3g/dL 62(78%) 41 (59%) RBV dose reduction 26 (33%) 25 (36%) Transfusion 13 (16%) 10 (14%) Topical steroid 16 (20%) 40 (57%) Rash Grade 4 0 2 (3%) Among BOC-treated patients, 10 (13%) patients stopped treatment due to AEs: severe depression (n=2), refractory insomnia (n=1), and profound lethargy (n=7, only 1 of which was associated with anemia), 3 patients were hospitalized for anaemia, infection in the setting of pancytopenia, and nausea and vomiting, respectively. Among TVR-treated patients, 7 (10%) discontinued due to AEs: 1 patient with severe anaemia (nadir Hb 79, 6 blood transfusions required in total) in the setting of cryoglobulinaemic myeloproliferative glomerulonephritis; 1 patient with grade 4 rash (DRESS syndrome); 1 cirrhotic patient developed a first hepatic decompensation event (spontaneous bacterial peritonitis requiring ICU admission); and 3 patients were hospitalised for symptomatic anaemia (fatigue and chest pain). 1 patient died from mucormycosis. 1 patient developed skin necrosis at a pIFN injection site 12 weeks after cessation of TVR and required a prolonged hospitalization . Conclusion: Treatment with PI-based triple therapy is challenging. Overall the rates of AEs were similar to those observed in the registration studies. The most common AE was anemia for both drugs; rash was more common in patients receiving TVR. Severe AEs were unusual. Careful monitoring of patients is recommended, particularly in the setting of advanced fibrosis. 1 Hézode C, et al. AASLD 2012